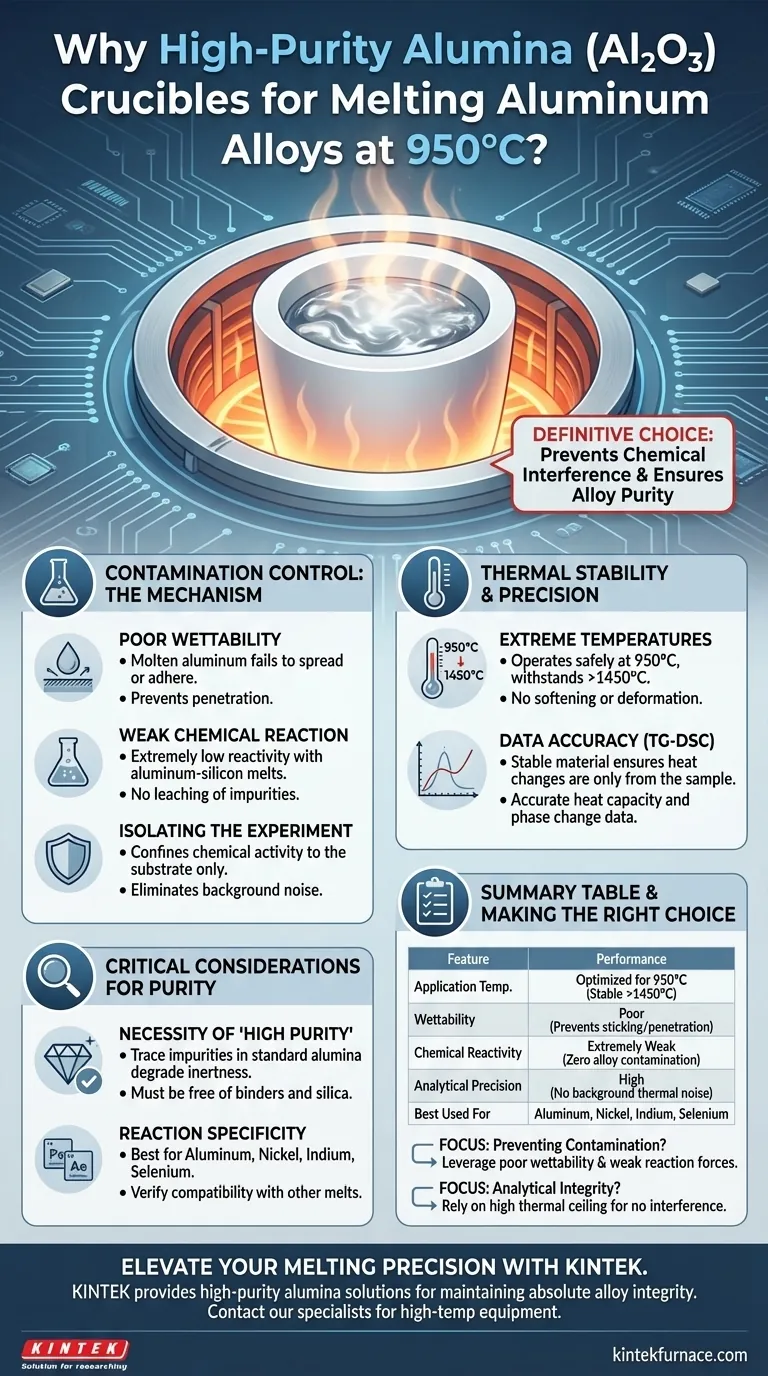
High-purity alumina (Al2O3) crucibles are the definitive choice for melting aluminum alloys at 950°C primarily because they prevent chemical interference. Their effectiveness stems from a lack of chemical affinity with aluminum-silicon melts, known as poor wettability, which prevents the molten metal from sticking to or degrading the crucible walls. This ensures the alloy remains pure and that any chemical reactions are confined strictly to the experimental substrate, not the container itself.
The core advantage of high-purity alumina is its ability to remain chemically inert at high heat; by resisting reaction and wetting, it isolates the molten alloy to guarantee the structural and chemical integrity of the final sample.

The Mechanism of Contamination Control
Poor Wettability
The primary driver for using alumina with aluminum alloys is poor wettability.
When aluminum-silicon alloys are melted at 950°C, the liquid metal struggles to spread across or adhere to the alumina surface. This physical property acts as a barrier, preventing the melt from penetrating the crucible pores.
Weak Chemical Reaction
At high temperatures, many container materials actively react with molten metals, leaching impurities into the melt.
High-purity alumina, however, exhibits an extremely weak reaction with aluminum alloys. This chemical stability ensures that the composition of the alloy does not drift during the melting process.
Isolating the Experiment
In scientific processing, you often want reactions to occur only at specific interfaces.
Because the crucible is inert, it forces all chemical activity to occur solely between the alloy and your specific experimental substrate. This eliminates "background noise" from the container, ensuring your results reflect only the variables you intended to test.
Thermal Stability and Precision
Withstanding Extreme Temperatures
While the target application is 950°C, high-purity alumina is capable of withstanding temperatures exceeding 1450°C.
Operating at 950°C places the material well within its safe performance window. This thermal headroom ensures the crucible maintains its structural rigidity and does not soften or deform during the melt.
Ensuring Data Accuracy
For analytical techniques like Thermogravimetric-Differential Scanning Calorimetry (TG-DSC), the container must not absorb or release heat via reaction.
Because alumina is stable, any heat changes recorded are solely from the sintering material or alloy. This guarantees that data regarding heat capacity and phase changes is accurate and free from interference.
Critical Considerations for Purity
The Necessity of "High Purity"
It is a common mistake to assume standard alumina performs identically to high-purity formulations.
The references explicitly emphasize "high-purity" Al2O3 because trace impurities in lower-grade crucibles can degrade inertness. If the alumina contains binders or silica additives, these can react with the aluminum melt, negating the benefits of the material.
Reaction Specificity
While alumina is inert to aluminum, Nickel, Indium, and Selenium, it is not universally inert to all chemistries.
The material is chosen specifically because it does not react with these defined sample groups. You must always verify that your specific melt composition shares this lack of affinity with Al2O3 before proceeding.
Making the Right Choice for Your Goal
To select the correct crucible for your high-temperature application, consider your primary objective:
- If your primary focus is preventing alloy contamination: Ensure you utilize high-purity Al2O3 to leverage its poor wettability and weak reaction forces against aluminum-silicon melts.
- If your primary focus is analytical data integrity: Rely on alumina's high thermal ceiling (>1450°C) to ensure no background thermal reactions interfere with your measurements.
By utilizing high-purity alumina, you transform the crucible from a potential variable into a reliable constant.
Summary Table:
| Feature | High-Purity Alumina (Al2O3) Performance |
|---|---|
| Application Temperature | Optimized for 950°C (Stable up to 1450°C+) |
| Wettability | Poor (Prevents molten metal from sticking/penetrating) |
| Chemical Reactivity | Extremely Weak (Ensures zero alloy contamination) |
| Analytical Precision | High (No background thermal noise for TG-DSC) |
| Best Used For | Aluminum, Nickel, Indium, and Selenium alloys |
Elevate Your Melting Precision with KINTEK
Don't let crucible contamination compromise your research or production quality. KINTEK provides high-purity alumina solutions designed to maintain the absolute integrity of your aluminum alloys. Backed by expert R&D and manufacturing, KINTEK offers a full range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside premium customizable lab high-temp furnaces to meet your unique thermal processing needs.
Ready to eliminate impurities? Contact our specialists today to find the perfect high-temperature equipment for your laboratory.
Visual Guide
References
- Hanka Becker, Andreas Leineweber. Reactive Interaction and Wetting of Fe‐ and Mn‐Containing, Secondary AlSi Alloys with Manganese Oxide Ceramic Filter Material for Fe Removal. DOI: 10.1002/adem.202500636
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
People Also Ask
- What are the advantages of glass tubing for heating applications? Key Benefits for Lab Efficiency
- How does a vacuum pump facilitate the synthesis process of rare earth-based halide electrolytes? Boost Chemical Purity
- Why is a high-performance vacuum pump system essential for magnesium purification? Achieve High Purity and Efficiency
- Why are high-purity alumina crucibles utilized for CsV3Sb5 crystal growth? Ensure Purity in Self-Flux Synthesis
- Why is a heating device with magnetic stirring required for Y2O3-MgO precursors? Ensure Perfect Particle Coating
- Why is a glovebox environment necessary for KBaBi synthesis? Protect Sensitive Raw Materials Today
- Why is an alundum crucible necessary for the melting and casting of FeAl alloys? Ensure Maximum Purity and Stability
- Why use R-type and K-type thermocouples for slag measurement? Optimize High-Temp Thermal Profiling and Modeling



















