At its core, a high-performance vacuum pump system is essential for purifying magnesium because it fundamentally alters the physical and chemical environment to favor the separation process. By drastically lowering the pressure inside a furnace, it reduces the energy required to vaporize the magnesium and simultaneously removes reactive atmospheric gases that would otherwise contaminate the final product.
The challenge in magnesium purification is separating the metal from its impurities at high temperatures without creating new ones. A high-performance vacuum system is the critical enabler, creating the ideal low-pressure, inert-like conditions for this to happen efficiently and with high purity.

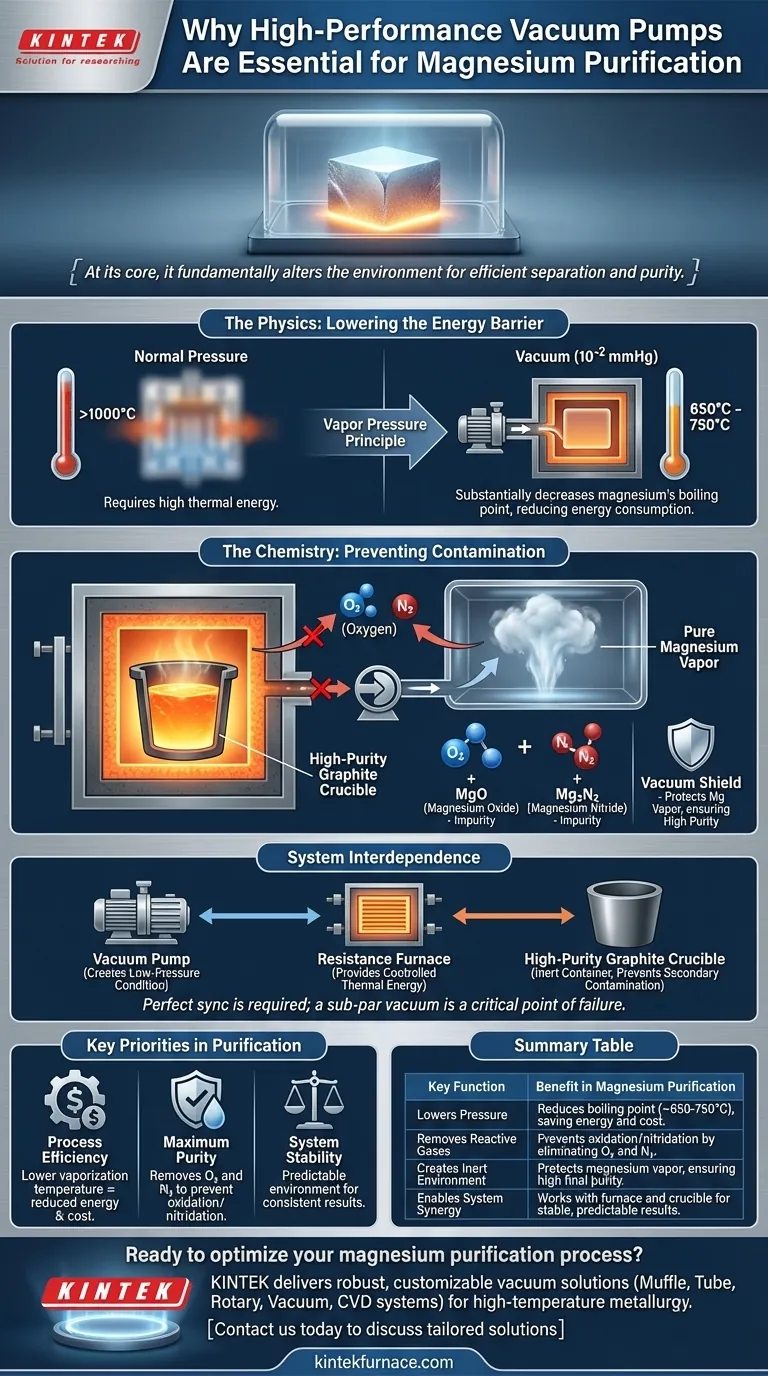
The Physics: Lowering the Energy Barrier
The Principle of Vapor Pressure
A substance boils or sublimates when the pressure of its vapor equals the pressure of the surrounding environment. Under normal atmospheric pressure, this requires a great deal of thermal energy.
Reducing Magnesium's Boiling Point
A vacuum pump works by removing gas molecules, drastically lowering the ambient pressure inside the furnace. By reducing the pressure to levels like 10⁻² mmHg, the temperature at which magnesium's vapor pressure is sufficient for it to boil or sublimate is substantially decreased.
The Practical Advantage
This process typically occurs in a resistance furnace operating between 650°C and 750°C. Without a vacuum, the temperatures required to vaporize magnesium would be far higher, making the process more expensive, energy-intensive, and demanding on equipment.
The Chemistry: Preventing Contamination
Magnesium's High Reactivity
At the high temperatures required for purification, magnesium vapor is extremely reactive. It will readily bond with any available, suitable elements.
The Threat of Atmospheric Gases
Standard air is composed primarily of nitrogen and oxygen. If these gases are present in the furnace, they pose a significant threat to the purity of the magnesium.
Oxidation and Nitridation
Hot magnesium vapor will instantly react with residual oxygen to form magnesium oxide and with nitrogen to form magnesium nitride. Both of these compounds are impurities that degrade the quality of the final product.
The Vacuum as a Protective Shield
By evacuating the furnace, the vacuum pump removes these reactive gases. This creates a chemically inert-like environment that protects the pure magnesium vapor as it travels from the crucible to the condensation area, ensuring its high purity is preserved.
Understanding System Interdependence
The Pump and the Furnace
The vacuum pump creates the necessary low-pressure condition, while the resistance furnace provides the controlled thermal energy. The two must work in perfect sync; the precise temperature is only effective because of the low pressure established by the pump.
The Role of the Crucible
While the vacuum prevents contamination from the atmosphere, an inert container like a high-purity graphite crucible is also essential. Graphite's stability prevents the molten magnesium from reacting with its container, avoiding secondary contamination.
The Consequence of a Weak Vacuum
A sub-par vacuum pump is a critical point of failure. It results in a higher boiling point (requiring more energy) and leaves behind residual reactive gases (causing contamination). Therefore, a "high-performance" system is non-negotiable for achieving both efficiency and purity.
Key Priorities in Magnesium Purification
- If your primary focus is process efficiency and cost: The vacuum pump's ability to lower the vaporization temperature is the most critical factor, as it directly reduces energy consumption.
- If your primary focus is achieving maximum product purity: The pump's effectiveness at removing oxygen and nitrogen to prevent oxidation and nitridation is paramount.
- If your primary focus is overall system stability: A high-performance vacuum system creates a predictable and controllable environment, allowing the furnace to operate optimally for consistent results.
Ultimately, the vacuum pump system is not just an auxiliary component; it is the cornerstone that makes modern, high-purity magnesium production technically and economically feasible.
Summary Table:
| Key Function | Benefit in Magnesium Purification |
|---|---|
| Lowers Pressure | Reduces boiling point (to ~650-750°C), saving energy and cost. |
| Removes Reactive Gases | Prevents oxidation/nitridation by eliminating O₂ and N₂. |
| Creates Inert Environment | Protects magnesium vapor, ensuring high final purity. |
| Enables System Synergy | Works with furnace and crucible for stable, predictable results. |
Ready to optimize your magnesium purification process?
A high-performance vacuum system is the cornerstone of achieving high-purity metal efficiently. At KINTEK, our expert R&D and manufacturing team delivers robust, customizable vacuum solutions—including Muffle, Tube, Rotary, Vacuum, and CVD systems—specifically designed to meet the demanding needs of high-temperature metallurgical processes.
Contact us today to discuss how we can tailor a vacuum furnace system to your unique purification requirements.
Visual Guide
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Why is a segmented PID control system necessary for lithium battery vacuum drying? Ensure Precision & Safety
- What is the primary function of the vacuum pump system in the magnesium powder evaporation process? Ensure High Purity & Efficiency
- What materials are used for the heating elements in a vacuum furnace? Choose the Right Element for Your High-Temp Needs
- What are the main technical requirements for vacuum pumps in vacuum sintering furnaces? Ensure Material Purity and Efficiency
- Why is a laboratory vacuum oven necessary for the processing of Nickel Oxide electrodes? Optimize Solvent Removal



















