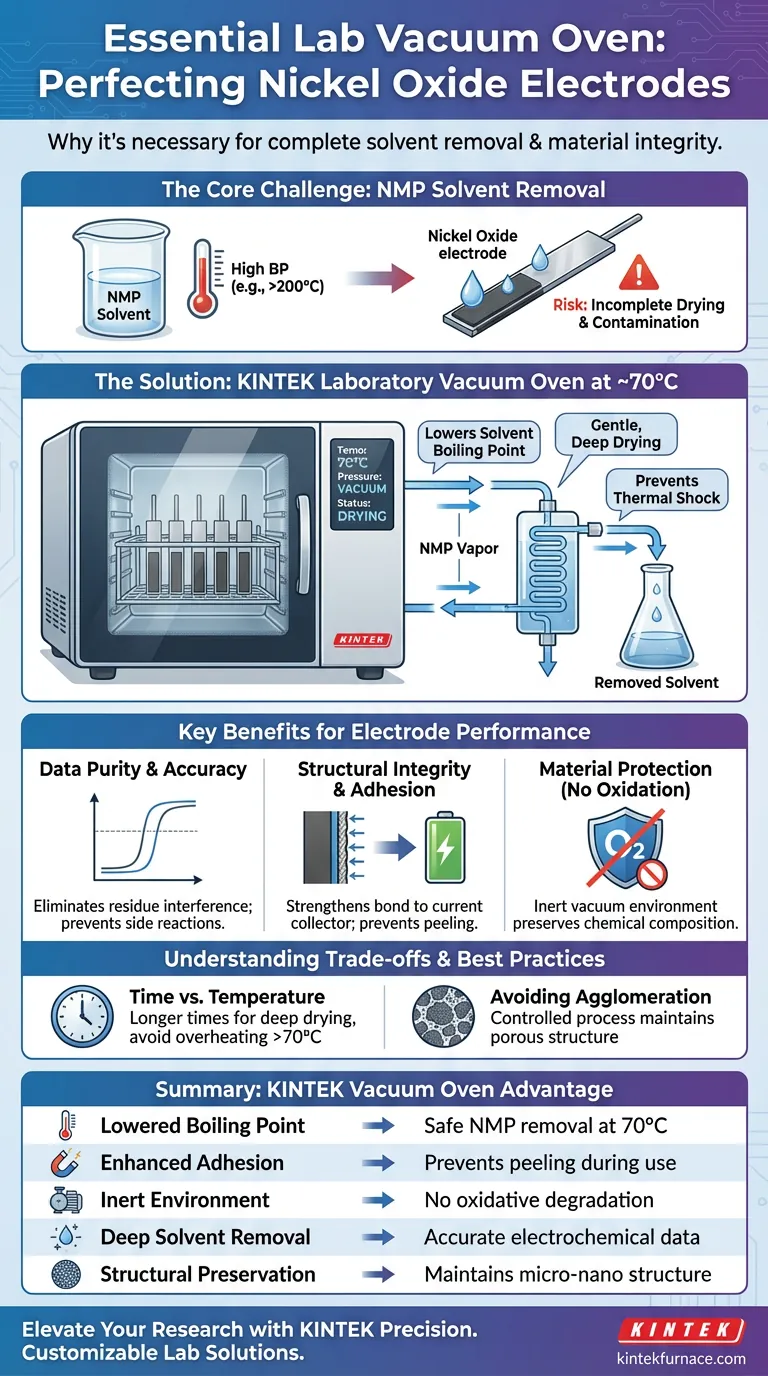
A laboratory vacuum oven is strictly necessary for processing Nickel Oxide electrodes to ensure the complete removal of high-boiling point organic solvents, most notably N-Methyl-2-pyrrolidone (NMP). Operating typically at a stable 70°C under vacuum, this process prevents the electrode active material from peeling off the current collector and eliminates solvent residues that would otherwise compromise electrochemical data accuracy.
Core Takeaway The vacuum oven solves the conflict between needing to remove stubborn solvents and needing to protect delicate materials. By lowering the boiling point of solvents like NMP, it achieves deep drying and strong structural adhesion without subjecting the Nickel Oxide to damagingly high temperatures or oxidative stress.

The Critical Role of Solvent Removal
Overcoming High Boiling Points
The primary challenge in processing Nickel Oxide electrodes is removing the solvent used in the coating slurry, such as N-Methyl-2-pyrrolidone (NMP).
NMP has a high boiling point, making it difficult to evaporate under standard atmospheric conditions without excessive heat.
The vacuum environment significantly lowers the boiling point of these solvents. This allows for their complete removal at a moderate temperature (around 70°C), ensuring the electrode is dry without requiring thermal extremes that could alter the material's properties.
Preventing Electrochemical Interference
Removing solvent residues is not just about drying; it is about data purity.
If NMP residues remain within the electrode structure, they can interfere with subsequent electrochemical measurements.
Vacuum drying eliminates these trace residues, preventing them from triggering side reactions or skewing the performance data. This ensures that the results reflect the true capabilities of the Nickel Oxide, rather than artifacts caused by contamination.
Structural Integrity and Performance
Maximizing Adhesion
A critical failure point in electrode manufacturing is the detachment of the active material from the current collector.
Vacuum drying increases the adhesion between the active Nickel Oxide material, conductive additives, and the current collector.
By thoroughly removing the solvent, the physical bond between these layers is strengthened. This prevents the electrode from peeling off when exposed to the electrolyte, ensuring mechanical stability during battery assembly and operation.
Protecting Against Oxidation
While the primary reference highlights solvent removal, the vacuum environment offers a secondary benefit: protection from oxidation.
Drying in a vacuum excludes oxygen, which prevents the oxidative degradation of the electrode materials during the heating process.
This preserves the chemical composition of the Nickel Oxide, ensuring that the material tested is chemically identical to the material synthesized.
Understanding the Trade-offs
Temperature vs. Time
While vacuum ovens allow for lower drying temperatures, they often require longer processing times to achieve "deep drying."
Rushing this process by arbitrarily increasing the temperature (e.g., beyond the recommended 70°C for this specific application) can lead to thermal shock or binder degradation.
The Risk of Agglomeration
Improper drying protocols can lead to the "hard agglomeration" of powders.
The vacuum process must be controlled to maintain the loose, porous characteristics of the material. If the drying is too aggressive, the fine micro-nano structure of the catalyst can be compromised, reducing the active surface area available for electrochemical reactions.
Making the Right Choice for Your Goal
To optimize your Nickel Oxide electrode processing, tailor your drying protocol to your specific objective:
- If your primary focus is Mechanical Stability: Prioritize a slow, steady vacuum ramp to maximize the physical bond and preventing peeling from the current collector.
- If your primary focus is Data Precision: Ensure the drying cycle is sufficiently long to remove all trace NMP residues, guaranteeing that your electrochemical measurements are free from solvent interference.
Success in electrode processing relies not just on heating, but on using vacuum pressure to remove solvents gently and completely.
Summary Table:
| Key Feature | Benefit for Nickel Oxide Electrodes |
|---|---|
| Lowered Boiling Point | Facilitates NMP removal at a safe 70°C, preventing material damage. |
| Enhanced Adhesion | Prevents active material from peeling off the current collector during use. |
| Inert Environment | Eliminates oxygen to prevent oxidative degradation during the drying cycle. |
| Deep Solvent Removal | Removes trace residues that cause side reactions or skewed electrochemical data. |
| Structural Preservation | Maintains porous micro-nano structures by avoiding aggressive thermal shock. |
Elevate Your Electrode Research with KINTEK Precision
Don't let solvent residues or material peeling compromise your electrochemical data. KINTEK provides industry-leading lab vacuum ovens specifically designed to handle delicate drying processes for Nickel Oxide and other advanced materials.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory requirements. Whether you are scaling up production or refining sensitive microstructures, our high-temperature solutions ensure consistent, high-purity results.
Ready to optimize your material processing? Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Mamta Bulla, Ajay Kumar Mishra. Natural resource-derived NiO nanoparticles via aloe vera for high-performance symmetric supercapacitor. DOI: 10.1038/s41598-024-57606-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How is temperature controlled during the heating process in a vacuum furnace? Unlock Precision for High-Integrity Processes
- Why is a vacuum drying oven necessary before the electrochemical testing of sodium-ion battery electrodes? Optimize SIBs
- What is the primary function of a laboratory vacuum oven in the synthesis of ABC triblock copolymers? Ensure Purity.
- What is the significance of using a vacuum diffusion annealing furnace for thermodynamic equilibrium studies in alloys?
- What is the function of computer-controlled systems in modern vacuum furnaces? Achieve Unwavering Precision & Repeatability
- What is vacuum carburizing and how does it work? Discover Advanced Case-Hardening for Superior Steel Components
- What processes can horizontal vacuum furnaces be used for? Unlock Versatile Thermal Applications
- Why is it necessary to pre-bake vacuum chambers to 10^-10 mbar? Ensure High RRR in Niobium Thin Films



















