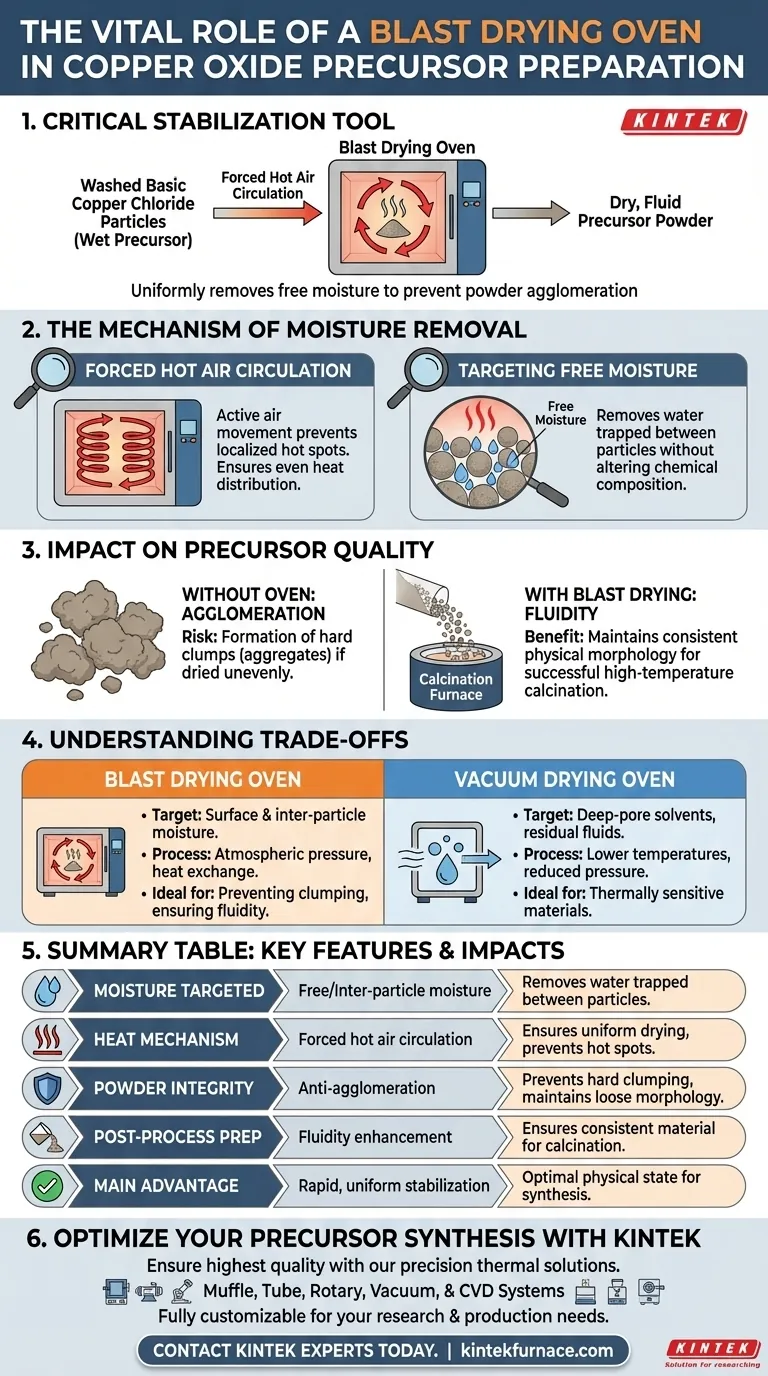
A laboratory blast drying oven functions as a critical stabilization tool in the preparation of copper oxide precursors. specifically for washed basic copper chloride particles. By utilizing forced hot air circulation, it performs constant-temperature drying to uniformly remove the free moisture located between particles.
The primary value of this process is the prevention of powder agglomeration. By effectively removing inter-particle moisture, the oven ensures the precursor powder retains the fluidity and consistent physical morphology necessary for successful high-temperature calcination.

The Mechanism of Moisture Removal
Forced Hot Air Circulation
The defining feature of a blast drying oven is its active air movement. Rather than relying on passive heating, the device forces hot air to circulate continuously around the sample.
This circulation ensures that heat is distributed evenly throughout the drying chamber. It prevents localized hot spots or cold pockets that could lead to uneven drying rates within the precursor batch.
Targeting Free Moisture
In the context of copper oxide precursors, specifically basic copper chloride, the goal is to remove free moisture. This is the water trapped between the physical particles of the powder after the washing phase.
By targeting this specific type of moisture, the oven prepares the material for the next stage of synthesis without altering its chemical composition prematurely.
Impact on Precursor Quality
Preventing Agglomeration
The most significant risk during the drying phase is the formation of hard clumps or aggregates. If the powder remains wet or dries unevenly, particles will stick together.
The blast drying oven mitigates this by drying the particles quickly and uniformly. This keeps the powder loose, preventing the severe agglomeration that would otherwise occur if the material remained in a wet state.
Ensuring Fluidity for Calcination
The step following drying is typically high-temperature calcination. For this process to work correctly, the input material must have good fluidity.
A blast-dried precursor maintains a consistent physical morphology. This loose, fluid state allows for better heat transfer and chemical reaction uniformity during the subsequent calcination process.
Understanding the Trade-offs
Blast Drying vs. Vacuum Drying
While a blast drying oven is ideal for removing surface and inter-particle moisture, it operates differently than a vacuum drying oven.
Vacuum ovens are generally preferred for removing residual solvents from deep within pores or when processing thermally sensitive materials at lower temperatures.
Efficiency Considerations
Blast drying relies on airflow and heat exchange at atmospheric pressure.
If your material contains deep-pore solvents or requires extremely low-temperature processing to prevent degradation, a blast oven may not be aggressive enough for deep extraction or gentle enough for sensitive compounds.
Making the Right Choice for Your Goal
To ensure the integrity of your copper oxide precursor, align your drying method with your specific material needs:
- If your primary focus is preventing clumping before calcination: Use a blast drying oven to remove inter-particle moisture and maintain powder fluidity.
- If your primary focus is removing deep-pore solvents: Consider a vacuum drying oven to extract residual fluids from within the material structure without high heat.
Success in precursor preparation relies on removing moisture without compromising the physical structure of the powder.
Summary Table:
| Feature | Blast Drying Oven Function | Impact on Copper Oxide Precursor |
|---|---|---|
| Moisture Targeted | Free/Inter-particle moisture | Removes water trapped between washed particles |
| Heat Mechanism | Forced hot air circulation | Ensures uniform drying and prevents hot spots |
| Powder Integrity | Anti-agglomeration | Prevents hard clumping; maintains loose morphology |
| Post-Process Prep | Fluidity enhancement | Ensures consistent material for high-temp calcination |
| Main Advantage | Rapid, uniform stabilization | Optimal physical state for subsequent synthesis |
Optimize Your Precursor Synthesis with KINTEK
Ensure the highest quality for your copper oxide precursors with our precision thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a wide range of laboratory equipment including high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems.
Whether you need to prevent agglomeration with uniform blast drying or require specialized vacuum systems for deep-pore solvent removal, our lab high-temperature furnaces and ovens are fully customizable to meet your unique research and production needs.
Contact KINTEK experts today to find the perfect drying solution for your lab.
Visual Guide
References
- Dengliang He, Shishan Xue. Integrated Alkali Gradient pH Control Purification of Acidic Copper-Containing Etching Waste Solution and Cu2(OH)3Cl Conversion-Calcination Process for High-Purity CuO. DOI: 10.3390/pr13092807
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does the analysis of optimized process paths assist in lab equipment selection? Expert Guide for Research Success
- Why is a vacuum oven required during the preparation of Al-CNTs/h-BN composites? Prevent Defects & Ensure Purity
- What is the primary function of multi-stage oxidation ovens? Secure High-Strength Carbon Fiber Stabilization
- How does a forced-air drying oven contribute to asphalt degradation? Accelerate Material Salt Erosion Simulation
- What role does Sodium Chloride (NaCl) play as a thermal buffer? Optimizing Si/Mg2SiO4 Composite Synthesis
- Why is precise control of carrier gas flow rates required for hydrochar activation? Optimize Carbon Yield & Purity
- What are the functions of a rotary evaporator and a vacuum drying oven in LTO sol-gel? Optimize Your Synthesis Process
- Why is a jaw crusher used for initial magnesite ore crushing? Maximize Efficiency & Protect Your Mill
















