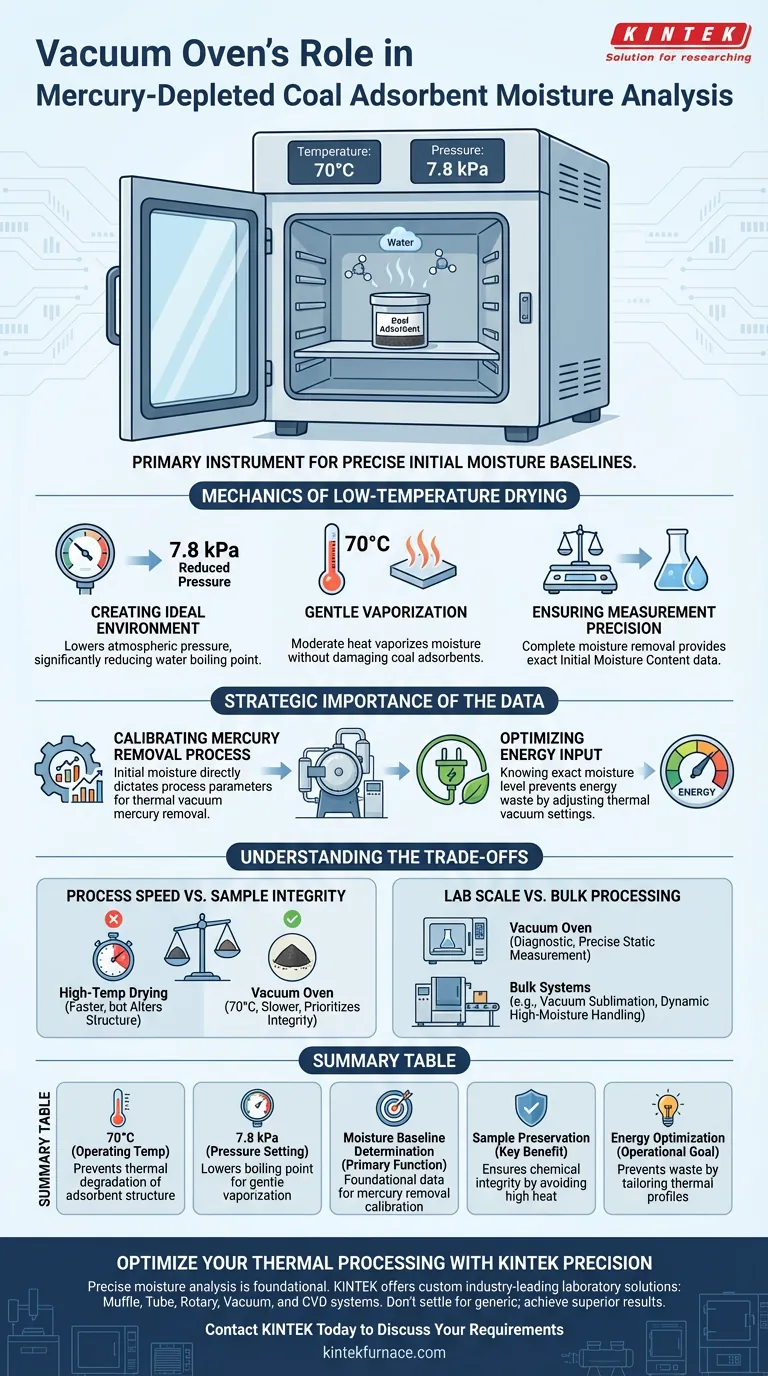
In the analysis of mercury-depleted coal adsorbents, the vacuum oven serves as the primary instrument for establishing precise initial moisture baselines. It operates by maintaining samples at a constant temperature of 70°C within a controlled, reduced-pressure environment of 7.8 kPa.
The vacuum oven facilitates the vaporization of moisture at significantly lower temperatures than standard drying methods. This process preserves the chemical integrity of the coal adsorbents while providing the critical data necessary to calibrate the subsequent thermal vacuum mercury removal process.
The Mechanics of Low-Temperature Drying
Creating the Ideal Environment
To determine moisture content accurately, the vacuum oven is set to a specific reduced pressure of 7.8 kPa. By lowering the atmospheric pressure surrounding the sample, the boiling point of the water trapped within the adsorbent is significantly reduced.
Gentle Vaporization
The oven maintains a steady temperature of 70°C. Because of the reduced pressure, this moderate heat is sufficient to vaporize moisture without exposing the coal adsorbents to the high temperatures typically required for drying at standard atmospheric pressure.
Ensuring Measurement Precision
This controlled environment allows for the complete removal of moisture. The resulting weight loss data provides an exact measurement of the initial moisture content, which is the foundational metric for all subsequent processing steps.
Strategic Importance of the Data
Calibrating the Mercury Removal Process
The data gathered from the vacuum oven is not merely for quality control; it is an operational prerequisite. The initial moisture content directly dictates the process parameters required for the thermal vacuum mercury removal phase.
Optimizing Energy Input
Knowing the exact moisture level prevents energy waste during the actual treatment. Operators can adjust the thermal vacuum settings to handle the specific water load of the batch, rather than applying a generic, potentially inefficient heating profile.
Understanding the Trade-offs
Process Speed vs. Sample Integrity
The Trade-off: Using a vacuum oven at 70°C is generally slower than high-temperature blast drying. The Reality: High temperatures could alter the structure of the coal adsorbent or prematurely volatilize other components. The vacuum method prioritizes the integrity of the sample over speed to ensure the moisture reading is isolated and accurate.
Lab Scale vs. Bulk Processing
The Trade-off: The vacuum oven is a diagnostic tool, not a bulk processing solution. The Reality: While the oven measures moisture, actual production lines often utilize equipment like vacuum sublimation furnaces with vibration feeding systems. These bulk systems are designed to handle high moisture levels (up to 20%) dynamically, whereas the vacuum oven is strictly for static, precise measurement.
Making the Right Choice for Your Goal
The role of the vacuum oven is to provide the data that steers the rest of your operation.
- If your primary focus is Process Calibration: Prioritize the accuracy of the vacuum oven reading, as this dictates the efficiency of your mercury removal parameters.
- If your primary focus is Sample Preservation: Adhere strictly to the 70°C limit to ensure the physical properties of the coal adsorbent remain unchanged during analysis.
Accurate moisture determination is the critical first step that ensures the safety and efficiency of the entire thermal treatment lifecycle.
Summary Table:
| Feature | Vacuum Oven Specification | Importance for Coal Adsorbents |
|---|---|---|
| Operating Temperature | 70°C | Prevents thermal degradation of the adsorbent structure. |
| Pressure Setting | 7.8 kPa (Reduced Pressure) | Lowers the boiling point of water for gentle vaporization. |
| Primary Function | Moisture Baseline Determination | Provides the foundational data for mercury removal calibration. |
| Key Benefit | Sample Preservation | Ensures chemical integrity by avoiding high-temperature drying. |
| Operational Goal | Energy Optimization | Prevents energy waste by tailoring thermal profiles to actual moisture levels. |
Optimize Your Thermal Processing with KINTEK Precision
Precise moisture analysis is the foundation of efficient material processing. KINTEK provides industry-leading laboratory solutions tailored for complex thermal treatments. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique research or production needs.
Whether you are analyzing mercury-depleted coal adsorbents or developing advanced materials, our high-temp furnaces ensure the accuracy and reliability your lab demands. Don't settle for generic heating profiles—achieve superior results with a custom solution.
Contact KINTEK Today to Discuss Your Requirements
Visual Guide
References
- Bagdaulet Kenzhaliyev, Xeniya Linnik. Preliminary Removal of Mercury from Depleted Coal Sorbents by Thermal Vacuum Method with Associated Extraction of Precious Metal Composite. DOI: 10.3390/jcs8090367
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What is the role of a laboratory oven in mushroom dehydration? Master Pre-Treatment for Precise Biochemical Analysis
- What role does a high-temperature furnace play in the chemical activation of carbon materials? Master KOH Activation
- Why are aluminum alloy castings subjected to high-temperature testing in an industrial blister oven? Reveal Defects
- Why is a constant temperature oven required for CoCrFeNiMn alloy powders? Ensure Superior Defect-Free Deposition
- What are the core process advantages of using a microwave reactor? Maximize Speed & Efficiency in Lab Characterization
- What is the importance of defining accurate heat transfer coefficients for slag? Master Thermal Stress Prediction
- How are high-temperature furnaces and precision balances used for alloy oxidation kinetics? Expert Analysis
- What is the significance of high vacuum base pressure in MoS2 sputtering? Ensuring Film Purity and Stoichiometry



















