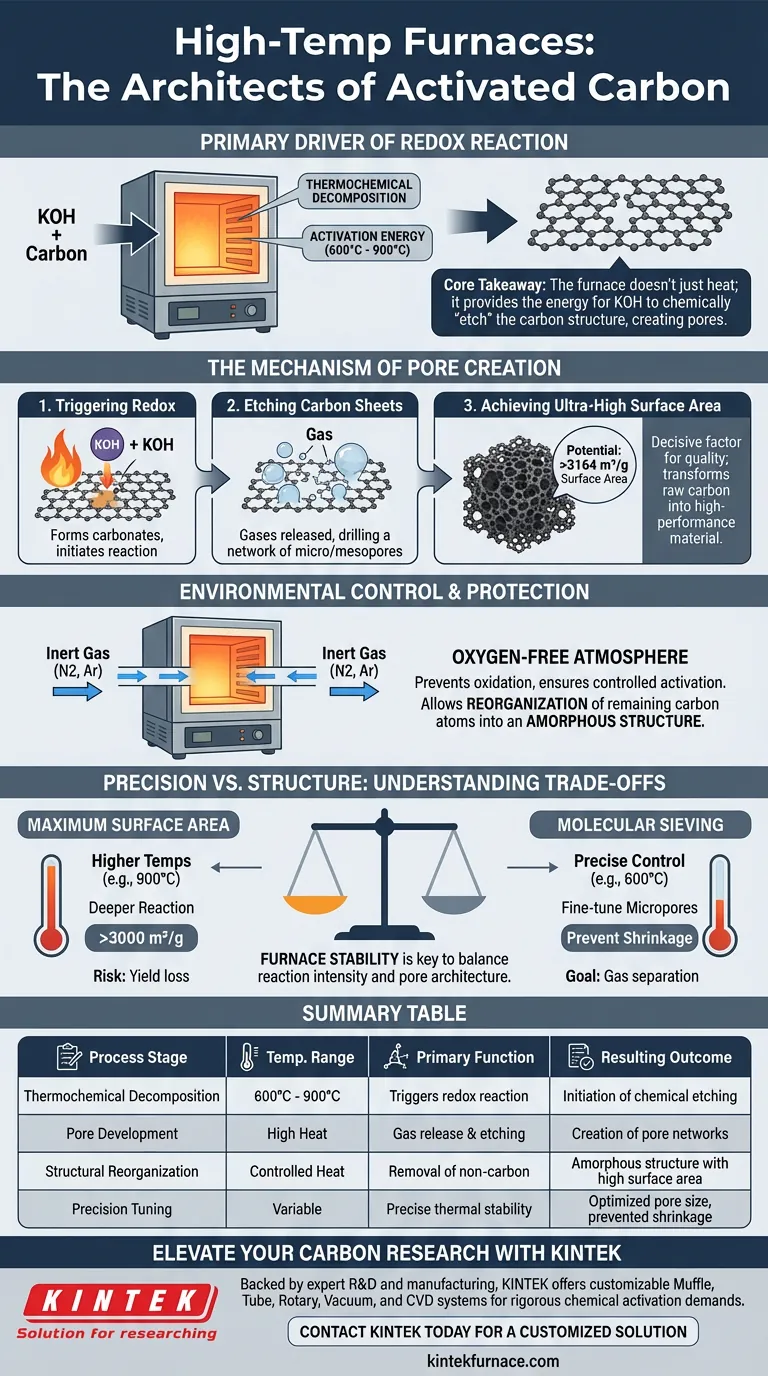
A high-temperature furnace acts as the primary driver of the redox reaction required to chemically activate carbon materials. By generating precise thermal energy, usually between 600°C and 900°C, the furnace forces potassium hydroxide (KOH) to react aggressively with the carbon skeleton, a process that is thermodynamically impossible at lower temperatures.
Core Takeaway: The furnace does not simply heat the material; it provides the activation energy for KOH to chemically "etch" the carbon structure. This reaction releases gases that drill a complex network of pores, transforming raw carbon into a material with ultra-high specific surface area.

The Mechanism of Pore Creation
Triggering the Redox Reaction
The primary function of the furnace is to initiate a thermochemical decomposition.
The heat causes the KOH to react with the carbon lattice, leading to the formation of carbonates (such as potassium carbonate).
Etching the Carbon Sheet
As this reaction progresses, it releases various gases within the material structure.
These expanding gases physically and chemically etch the carbon sheets, creating a vast, interconnected network of micropores and mesopores.
Achieving Ultra-High Surface Area
This etching process is the decisive factor in determining the final quality of the material.
Without the high-temperature environment, the material remains standard carbon; with it, the specific surface area can reach ultra-high values, potentially exceeding 3164 m²/g.
Environmental Control and Protection
Maintaining an Inert Atmosphere
Beyond temperature, the furnace (typically a tube furnace) manages the chemical environment using a continuous flow of inert gas, such as nitrogen or argon.
This creates an oxygen-free atmosphere, ensuring the carbon undergoes controlled activation rather than simply burning away (oxidation).
Carbon Atom Reorganization
The controlled heat induces the removal of non-carbon atoms and allows for the reorganization of the remaining carbon structure.
This results in a highly amorphous structure, which is essential for maximizing the material's reactive surface.
Understanding the Trade-offs: Precision vs. Structure
The Risk of Pore Shrinkage
While high heat is necessary for activation, the specific temperature chosen (e.g., 600°C vs. 900°C) dictates the final pore architecture.
The precision of temperature control is critical; incorrect temperatures can lead to micropore shrinkage, altering the material's ability to act as a molecular sieve.
Balancing Reaction Intensity
A higher temperature drives a deeper reaction and higher surface area, but it must be balanced against yield loss.
The furnace provides the stability required to maintain this balance, allowing for the precise tuning of pore sizes for specific applications like gas separation.
Making the Right Choice for Your Goal
To maximize the effectiveness of your activation process, align your furnace parameters with your specific material objectives:
- If your primary focus is Maximum Surface Area: Utilize high temperatures to drive a complete redox reaction, ensuring deep etching for surface areas exceeding 3000 m²/g.
- If your primary focus is Molecular Sieving: Prioritize the precision of the furnace's temperature control to fine-tune micropore size and prevent unwanted shrinkage or pore collapse.
The furnace is not merely a heating element; it is the architect of the carbon's internal geometry.
Summary Table:
| Process Stage | Temperature Range | Primary Function | Resulting Outcome |
|---|---|---|---|
| Thermochemical Decomposition | 600°C - 900°C | Triggers redox reaction between KOH and carbon | Initiation of chemical etching |
| Pore Development | High Heat | Gas release and carbon sheet etching | Creation of micro/mesopore networks |
| Structural Reorganization | Controlled Heat | Removal of non-carbon atoms in inert gas | Amorphous structure with high surface area |
| Precision Tuning | Variable | Precise thermal stability | Optimized pore size and prevented shrinkage |
Elevate Your Carbon Research with KINTEK
Precise temperature control and inert atmosphere stability are the difference between standard carbon and high-performance activated materials. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous demands of chemical activation. Whether you are aiming for surface areas exceeding 3000 m²/g or specific molecular sieving architectures, our high-temp furnaces provide the thermal accuracy your lab requires.
Ready to optimize your activation process? Contact KINTEK today for a customized solution.
Visual Guide
References
- Ewa Mijowska, Klaudia Maślana. Highly Porous Carbon Flakes Derived from Cellulose and Nickel Phosphide Heterostructure towards Efficient Electrocatalysis of Oxygen Evolution Reaction. DOI: 10.3390/molecules29020352
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a graphite furnace better than a flame in AAS? Unlock Trace-Level Detection for Your Lab
- How does controlled thermal treatment affect delta-MnO2? Optimize Porosity & Surface Area for Better Battery Performance
- How does the extended isothermal calcination in a furnace contribute to crystalline quality? Boost Material Purity
- How does a vacuum pressure impregnation tank achieve deep treatment? Master Advanced Wood Modification Methods
- How does a vacuum drying oven provide superior performance for MoS2/C powders? Preserve Purity and Nanostructure
- How does a symmetric suction design improve steel wire heat treatment? Achieve Uniform Salt Flow and Sorbite Quality
- What is the primary role of high-temperature thermal simulation systems in steel testing? Optimize Industrial Processes
- What is the function of a Teflon-lined autoclave in CuO nanoparticle synthesis? Achieve Precision Lab Results



















