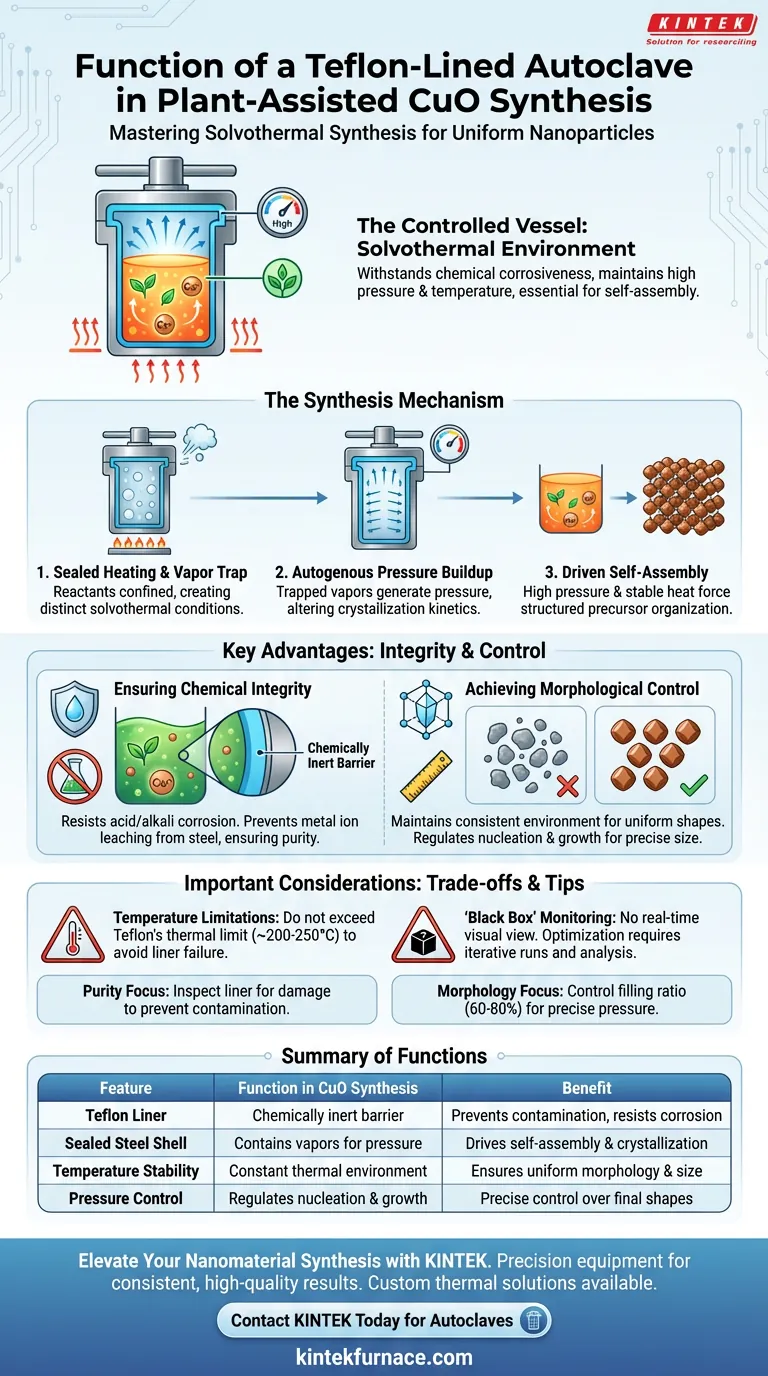
A Teflon-lined autoclave acts as a specialized containment vessel designed to create a controlled solvothermal environment for the synthesis of copper oxide (CuO) nanoparticles. Its primary function is to withstand the chemical corrosiveness of the reaction solution while maintaining high pressure and temperature, conditions that are essential for the self-assembly of uniform nanostructures.
Core Takeaway Standard heating methods often fail to produce uniform nanoparticles because they lack pressure control and introduce impurities from the container. A Teflon-lined autoclave solves this by generating autogenous pressure within a chemically inert chamber, forcing the copper precursors to crystallize into pure, highly ordered shapes.

Creating the Optimal Reaction Environment
The Mechanism of Solvothermal Synthesis
The autoclave facilitates a process known as solvothermal synthesis. By sealing the reactants within a confined space and heating them, the device creates an environment distinct from open-air boiling.
Generating Autogenous Pressure
As the solution heats up inside the sealed steel shell, vapors are trapped. This builds autogenous pressure (pressure generated by the reaction itself), which significantly alters the crystallization kinetics of the copper oxide.
Facilitating Self-Assembly
The combination of high pressure and stable heat drives the self-assembled growth of precursors. This forces the copper ions and plant-derived phytochemicals to organize into structured lattices rather than random aggregates.
Ensuring Chemical Integrity
Resisting Chemical Corrosion
The synthesis of CuO, particularly when assisted by plant extracts, involves reactive chemical solutions. The Teflon lining is chemically inert, protecting the stainless steel outer shell from acid or alkali corrosion during the reaction.
Preventing Contamination
Without the Teflon barrier, ions from the steel casing could leach into the solution. The lining ensures that the final copper oxide nanoparticles are free from metallic impurities derived from the reactor itself.
Achieving Morphological Control
Uniformity of Nanostructures
The autoclave maintains a consistent environment throughout the synthesis duration. This stability ensures that the morphology (shape and structure) of the resulting nanoparticles is uniform, rather than irregular or varied.
Controlled Crystal Growth
By regulating the internal pressure and temperature, the autoclave allows for precise control over the nucleation and growth phases. This results in well-defined particle sizes and shapes tailored to specific applications.
Understanding the Trade-offs
Temperature Limitations
While Teflon is highly resistant to chemicals, it has a lower melting point than steel. You must ensure your synthesis temperature does not exceed the thermal stability limit of the Teflon liner (typically around 200°C to 250°C), or the liner will deform and fail.
"Black Box" Monitoring
Because the autoclave is a sealed steel unit, you cannot visually monitor the reaction in real-time. Optimization requires an iterative process of running the synthesis, cooling, and analyzing the results, rather than adjusting parameters on the fly.
Making the Right Choice for Your Goal
To maximize the effectiveness of your synthesis, align your autoclave usage with your specific research objectives:
- If your primary focus is Purity: Ensure the Teflon liner is inspected for scratches or wear before every use to prevent trace metal contamination from the outer steel shell.
- If your primary focus is Morphology: Precisely control the filling ratio of the autoclave (usually 60-80%), as the volume of liquid directly impacts the internal pressure generated during heating.
By mastering the pressure and temperature variables within this vessel, you turn a simple mixture into high-quality, uniform nanomaterials.
Summary Table:
| Feature | Function in CuO Synthesis | Benefit |
|---|---|---|
| Teflon Liner | Provides a chemically inert barrier | Prevents metallic contamination and resists corrosion |
| Sealed Steel Shell | Contains vapors to build autogenous pressure | Drives self-assembly and crystallization of nanostructures |
| Temperature Stability | Maintains constant thermal environment | Ensures uniform particle morphology and size distribution |
| Pressure Control | Regulates nucleation and growth phases | Allows for precise control over final nanoparticle shapes |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision in nanoparticle morphology requires equipment that handles extreme pressure without compromising purity. Backed by expert R&D and manufacturing, KINTEK offers high-performance Teflon-lined hydrothermal autoclaves, Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable for your unique lab requirements.
Whether you are refining plant-assisted synthesis or scaling up advanced chemical research, our team provides the reliable thermal solutions you need to ensure consistent, high-quality results.
Ready to optimize your synthesis process? Contact us today to find the perfect lab equipment for your needs!
Visual Guide
References
- Muhammad Farooq, Magdi E. A. Zaki. Phytoassisted synthesis of CuO and Ag–CuO nanocomposite, characterization, chemical sensing of ammonia, degradation of methylene blue. DOI: 10.1038/s41598-024-51391-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What are the main advantages of crucible furnaces? Unmatched Flexibility for Small-Scale Melting
- What is the purpose of introducing high-purity argon gas into an immersed probe? Enhance Melt Analysis Accuracy
- What mechanism causes the formation of micro-cracks in zinc clinker during microwave heating? Boost Leaching Efficiency
- Why is determining the hypercooling limit necessary when measuring the heat of fusion? Optimize Your Material Research
- Why is precise temperature control in an aging oven critical for ZK61 alloys? Master the 175°C Pre-aging Threshold
- Why is a high-pressure stainless steel autoclave required for activated carbon? Unlock High-Performance Carbon Synthesis
- What are the primary applications of a constant temperature drying oven? Master Biochar Selenium-Modification
- Why is it necessary to use an annealing furnace at 350°C for three hours? Ensuring Glass Stability and Clarity



















