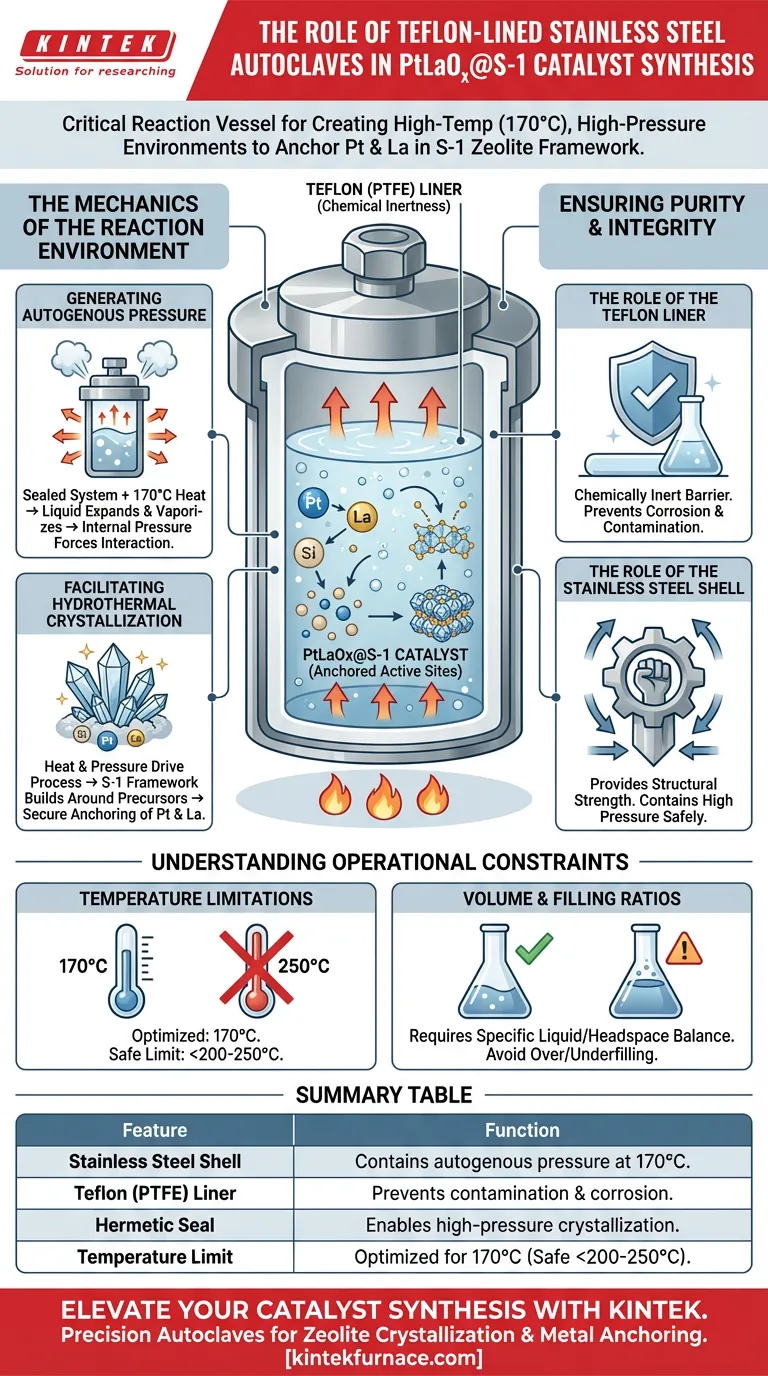
A Teflon-lined stainless steel autoclave serves as the critical reaction vessel that enables the hydrothermal synthesis of PtLaOx@S-1 catalysts. It creates a hermetically sealed, high-temperature (170 °C) environment where autogenous pressure drives the crystallization process, effectively anchoring platinum and lanthanum species within the silicalite-1 zeolite framework.
Core Insight: The autoclave’s specific value lies in its dual functioning: the stainless steel shell provides the structural strength to contain high pressure, while the Teflon liner ensures chemical inertness to prevent contamination, creating the precise conditions needed for bimetallic active sites to form inside the zeolite structure.

The Mechanics of the Reaction Environment
Generating Autogenous Pressure
The autoclave functions as a closed system. As the temperature rises to 170 °C, the liquid inside expands and creates vapor.
Because the vessel is sealed, this generates "autogenous pressure"—internal pressure created by the reactants themselves. This pressure is essential for forcing the silicon, platinum, and lanthanum sources into the necessary interaction.
Facilitating Hydrothermal Crystallization
The combination of heat and pressure drives the hydrothermal crystallization process.
This environment allows the silicalite-1 (S-1) zeolite framework to build itself around the metal precursors. This results in the Pt and La species being securely anchored within the zeolite structure rather than just sitting on the surface.
Ensuring Catalyst Purity and Integrity
The Role of the Teflon (PTFE) Liner
The internal Teflon lining provides a chemically inert barrier between the reaction mixture and the steel shell.
Hydrothermal synthesis often involves acidic or alkaline solutions that would corrode bare metal. The Teflon liner prevents this corrosion, ensuring that the final PtLaOx@S-1 catalyst is not contaminated by iron or other metals leaching from the autoclave walls.
The Role of the Stainless Steel Shell
While Teflon offers chemical resistance, it lacks the mechanical strength to withstand high internal pressures on its own.
The stainless steel outer shell provides the necessary structural integrity. It contains the expanding forces generated at 170 °C, allowing the reaction to proceed safely without the vessel rupturing or deforming.
Understanding Operational Constraints
Temperature Limitations
While effective for this specific synthesis (170 °C), Teflon liners have thermal limits.
They typically degrade or deform if temperatures exceed 200–250 °C. For the synthesis of PtLaOx@S-1, the operating temperature is well within the safe range, but precise temperature control is vital to maintain the liner's integrity.
Volume and Filling Ratios
The "sealed environment" relies on a specific balance of liquid and headspace.
Overfilling the autoclave can lead to dangerous pressure spikes that exceed the steel shell's rating. Underfilling may fail to generate the autogenous pressure required to properly anchor the bimetallic sites.
Making the Right Choice for Your Goal
When utilizing this equipment for catalyst synthesis, consider your primary objective:
- If your primary focus is Structural Stability: Ensure the stainless steel casing is defect-free to safely maintain the autogenous pressure required for the zeolite framework crystallization.
- If your primary focus is Chemical Purity: Inspect the Teflon liner for scratches or wear before use to guarantee zero interaction between the metal vessel and your precursors.
By balancing pressure containment with chemical inertness, this apparatus ensures the successful creation of high-performance anchored catalysts.
Summary Table:
| Feature | Function in PtLaOx@S-1 Synthesis |
|---|---|
| Stainless Steel Shell | Provides structural strength to contain autogenous pressure at 170 °C. |
| Teflon (PTFE) Liner | Offers chemical inertness to prevent iron contamination and corrosion. |
| Hermetic Seal | Enables high-pressure crystallization for anchoring metals in the S-1 framework. |
| Temperature Limit | Optimized for 170 °C (Safe operating range below 200-250 °C). |
Elevate Your Catalyst Synthesis with KINTEK
Precision in hydrothermal synthesis requires equipment that balances high-pressure safety with absolute chemical purity. KINTEK provides industry-leading Teflon-lined stainless steel autoclaves designed specifically for sensitive zeolite crystallization and metal anchoring processes.
Backed by expert R&D and manufacturing, KINTEK offers a full suite of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside customizable lab high-temp furnaces tailored to your unique research needs. Ensure the integrity of your bimetallic active sites with our reliable, high-performance solutions.
Ready to optimize your lab's performance? Contact our technical experts today to find the perfect customizable solution for your catalyst research.
Visual Guide
References
- Guilin Wei, Xingwen Feng. Embedding Monodisperse LaO <i> <sub>x</sub> </i> Into Pt Nanoclusters for Ultra‐Stable and Efficient Hydrogen Isotope Oxidation. DOI: 10.1002/advs.202504224
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why is electromagnetic stirring technology employed during the melting process of Titanium-Copper (Ti–Cu) alloys?
- Why is precise nitrogen flow critical for AlN nanofibers? Mastering High-Performance Nitridation Results
- Why is dual heat treatment required for SnO2 nanoparticles? Optimize Oxidation for Superior Performance
- Why must a laboratory drying furnace undergo a preheating stage? Ensure Accurate Mango Seed Drying Data
- How does the ramp rate affect LDO properties? Master Rapid Thermal Control for 69% More Efficiency
- Why is a heating system with closed-loop feedback essential for TL analysis? Precision Tips for High-Accuracy Kinetics
- Why are a blast drying oven and a freeze dryer both necessary for GO nanofibers? Essential Drying Synergy
- What role does pack media play in the solid-state powder boriding process? Enhance Metal Hardness at High Temperatures



















