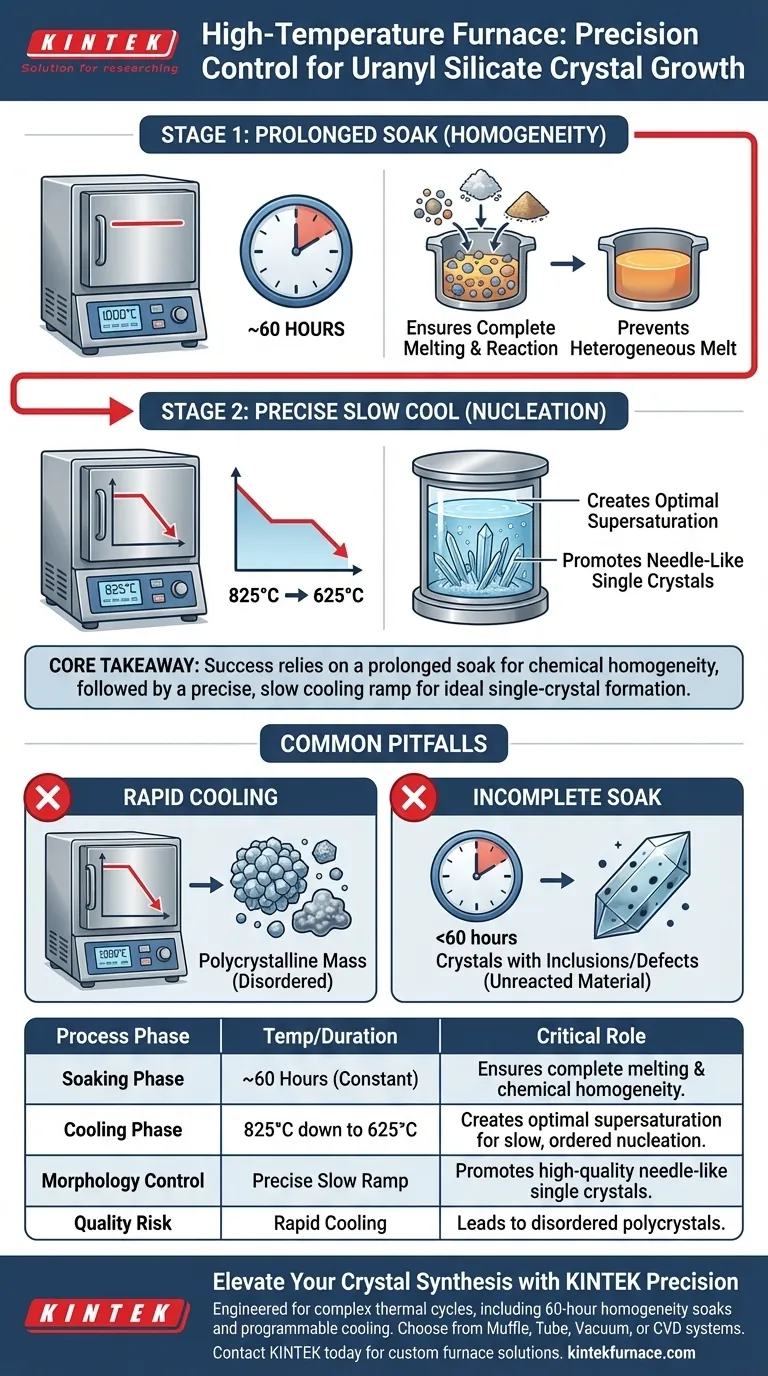
High-temperature furnaces with precision program temperature control serve as the definitive environment for synthesizing high-quality uranyl silicate single crystals. They execute complex thermal profiles that ensure the complete melting of raw materials followed by a strictly regulated cooling process to drive slow, ordered nucleation.
Core Takeaway Success in growing uranyl silicate crystals relies on a two-stage thermal strategy: a prolonged constant-temperature soak to achieve chemical homogeneity, followed by a precise, slow cooling ramp to create the ideal supersaturation environment for single-crystal formation.

Achieving Chemical Homogeneity
To cultivate high-quality crystals, the starting material must be perfectly uniform. Precision furnaces enable this through rigorous control of the heating phase.
The Role of Constant-Temperature Soaking
A standard high-temperature furnace is not enough; the system must maintain a specific constant temperature for an extended period.
For uranyl silicates, this often involves a soaking period of roughly 60 hours.
Ensuring Complete Reaction
This extended duration is critical for the thermodynamics of the mixture. It ensures that all raw materials are fully melted and have thoroughly reacted with one another.
Without this precision hold time, the melt remains heterogeneous, leading to inconsistent crystal growth later in the process.
Controlling Nucleation through Cooling
Once the materials are fully reacted, the transition from liquid to solid determines the final structure. This is where programmable temperature control becomes the primary driver of quality.
Establishing the Supersaturation Environment
The cooling process must be slow and deliberate, typically moving from 825°C down to 625°C.
This controlled descent creates an optimal supersaturation environment. It allows the dissolved materials to precipitate out of the solution gradually rather than solidifying instantly.
Promoting Needle-Like Morphology
The specific goal for uranyl silicates is often the formation of high-quality needle-like single crystals.
Precision cooling prevents the formation of unwanted polycrystalline structures or amorphous products (glass), which occur when the temperature drops too rapidly or unevenly.
Common Pitfalls to Avoid
While the furnace provides the capability, the thermal profile itself involves trade-offs that must be managed.
The Risk of Rapid Cooling
If the furnace cannot maintain a slow, steady ramp-down, the system enters a state of high supersaturation too quickly.
This triggers rapid nucleation at many points simultaneously, resulting in a mass of small, disordered polycrystals rather than a single, large, ordered crystal.
Incomplete Soaking
Cutting the 60-hour soaking time short to save energy or time is a frequent error.
If the melt is not fully homogenized before cooling begins, the resulting crystals will likely contain inclusions or defects derived from unreacted raw materials.
Making the Right Choice for Your Goal
When configuring your thermal profile for uranyl silicate preparation, align your parameters with your specific structural requirements.
- If your primary focus is crystal clarity and order: Prioritize the precision of the cooling ramp (825°C to 625°C) to ensure a stable supersaturation environment for needle-like growth.
- If your primary focus is material homogeneity: Ensure your programming includes the full 60-hour soaking duration to guarantee complete melting and reaction of the raw inputs.
Precision temperature control is not merely a feature; it is the fundamental mechanism that dictates whether you produce a high-value single crystal or a disordered amorphous solid.
Summary Table:
| Process Phase | Temperature/Duration | Critical Role for Uranyl Silicate |
|---|---|---|
| Soaking Phase | ~60 Hours (Constant) | Ensures complete melting and chemical homogeneity of raw materials. |
| Cooling Phase | 825°C down to 625°C | Creates optimal supersaturation for slow, ordered nucleation. |
| Morphology Control | Precise Slow Ramp | Promotes the growth of high-quality needle-like single crystals. |
| Quality Risk | Rapid Cooling | Leads to disordered polycrystals instead of single crystals. |
Elevate Your Crystal Synthesis with KINTEK Precision
Achieving the perfect needle-like morphology in uranyl silicate crystals requires absolute thermal stability and programmable accuracy. KINTEK provides the cutting-edge high-temperature furnace technology needed to master your soaking and cooling profiles.
Why partner with KINTEK?
- Expert R&D: Systems engineered for complex thermal cycles like the 60-hour homogeneity soak.
- Versatile Solutions: Choose from Muffle, Tube, Vacuum, or CVD systems tailored for laboratory precision.
- Customizable Performance: Fully programmable temperature controllers to manage delicate supersaturation environments.
Whether you are growing single crystals or developing advanced materials, our furnaces deliver the reliability your research demands. Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Еvgeny V. Nazarchuk, Dmitri O. Charkin. A novel microporous uranyl silicate prepared by high temperature flux technique. DOI: 10.1515/zkri-2024-0121
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why are high-temperature vacuum or atmosphere furnaces used for annealing metal silicide? Unlock Peak Thermal Stability
- What makes inert atmosphere furnaces different from standard tube furnaces? Key Benefits for Material Protection
- Why Calcination of Carbon-Supported Nickel Catalysts Needs Inert Gas? Protect Your Support Structure
- What is the role of an Argon gas environment in sintering Boron Carbide? Achieve High Density and Prevent Oxidation
- What are the dual functions of the inner cover in a bell-type annealing furnace? Heat Transfer and Protective Sealing
- Can the reducing atmosphere be replaced with other gaseous mediums? Explore Advanced Surface Engineering Solutions
- What types of furnaces are specially designed for processing in inert atmospheres? Explore Sealed Systems for Oxidation-Free Results
- Why is a continuous argon flow necessary during the thermal treatment of graphite? Achieve 2400 °C Ultra-Deep Purification



















