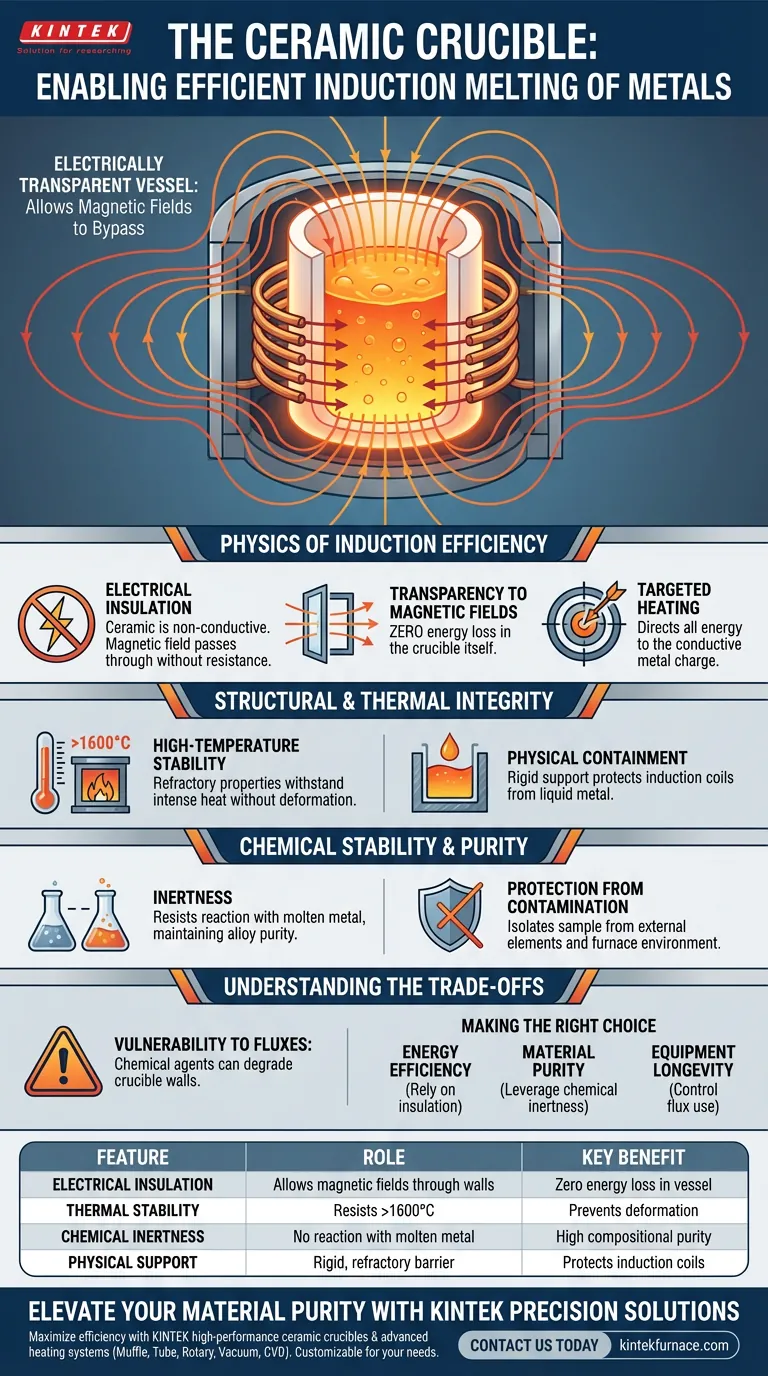
A ceramic crucible functions as the electrically transparent vessel required for the efficient induction melting of metals. Unlike conductive containers, a ceramic crucible (typically made of alumina or corundum) allows alternating magnetic fields to pass directly through its walls without absorbing energy, ensuring the heat is generated solely within the metal charge inside.
The ceramic crucible is critical to induction efficiency because it is effectively "invisible" to the magnetic field. It acts as a stable physical barrier that contains the molten metal and withstands extreme temperatures, while allowing electromagnetic energy to bypass the container and couple directly with the metal.
The Physics of Induction Efficiency
Electrical Insulation
The defining characteristic of a ceramic crucible in this context is that it is electrically non-conductive. This insulation is paramount for the induction process to work correctly.
Transparency to Magnetic Fields
Because the material is insulating, the alternating magnetic field generated by the induction coil passes through the crucible walls without resistance. This ensures there is zero energy loss within the crucible itself.
Targeted Heating
By allowing the magnetic field to penetrate freely, the system directs all energy into the conductive metal charge inside. The crucible remains a passive container, while the metal becomes the active heating element.
Structural and Thermal Integrity
High-Temperature Stability
Ceramic materials, such as alumina (corundum), possess refractory properties that allow them to withstand intense heat without melting or deforming. While aluminum melts at approximately 750°C, high-grade corundum ceramics can maintain structural integrity at temperatures exceeding 1600°C.
Physical Containment
The crucible provides the necessary rigid support to hold the heavy liquid metal. It acts as a robust physical barrier, preventing the molten charge from coming into contact with the delicate induction coils or the furnace lining.
Chemical Stability and Purity
Inertness
A major advantage of using high-quality ceramic is its chemical inertness. It resists reacting with the molten metal, which is vital for maintaining the compositional purity of the alloy being produced.
Protection from Contamination
By acting as a neutral barrier, the crucible isolates the sample from external elements. This prevents cross-contamination between the melt and the surrounding furnace environment.
Understanding the Trade-offs
Vulnerability to Fluxes
While ceramics are robust against heat, they are chemically sensitive to certain additives. The introduction of fluxing agents can be detrimental to the crucible's structure.
Chemical Erosion
Using inappropriate fluxes, or using flux with incompatible metal-crucible combinations, can trigger aggressive chemical reactions. This degrades the crucible walls, significantly shortening its useful lifespan and potentially leading to containment failure.
Making the Right Choice for Your Goal
To ensure your induction melting process is safe and efficient, align your crucible usage with your specific operational priorities:
- If your primary focus is energy efficiency: Rely on the ceramic's electrical insulation to maximize the magnetic coupling with your metal charge.
- If your primary focus is material purity: Leverage the chemical inertness of alumina/corundum to prevent reactions between the vessel and the melt.
- If your primary focus is equipment longevity: strictly control or eliminate the use of fluxing agents to prevent rapid chemical degradation of the crucible.
The ceramic crucible is not merely a container; it is a precision component that enables the direct transfer of energy while safeguarding the purity of your melt.
Summary Table:
| Feature | Role in Induction Melting | Key Benefit |
|---|---|---|
| Electrical Insulation | Allows magnetic fields to pass through the walls | Zero energy loss in the vessel itself |
| Thermal Stability | Resists temperatures exceeding 1600°C | Prevents deformation during high-heat melting |
| Chemical Inertness | Does not react with the molten metal charge | Ensures high compositional purity of alloys |
| Physical Support | Acts as a rigid, refractory barrier | Protects induction coils from liquid metal contact |
Elevate Your Material Purity with KINTEK Precision Solutions
Maximize your induction melting efficiency with KINTEK’s high-performance ceramic crucibles and advanced heating systems. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique metallurgical needs.
Whether you are processing precious alloys or researching advanced ceramics, our team provides the technical expertise to ensure your operations are safe, efficient, and contamination-free. Contact us today to optimize your lab's high-temperature processes!
Visual Guide
References
- Pablo Garcia-Michelena, Xabier Chamorro. Numerical Simulation of Free Surface Deformation and Melt Stirring in Induction Melting Using ALE and Level Set Methods. DOI: 10.3390/ma18010199
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the function of a vacuum induction furnace? Enhancing Purity in Silicon Steel Melting
- Why is an argon gas shielding environment necessary for the stir casting system? Ensure High-Purity Al2214 Composites
- What role does a vacuum arc melting furnace play in Ti-6Al-7Nb-xTa alloys? Precision Melting & Purity
- What is the role of a vacuum arc furnace in the synthesis of AlCrFeNi HEAs? Achieve High-Purity Material Homogeneity
- What role does a vacuum induction melting furnace play in CoCrFeMnNi production? Ensure Purity and Homogeneity
- What crucible materials are used in IGBT induction melting furnaces for different metals? Select the Right Crucible for a Clean, High-Quality Melt
- What is an IGBT induction melting furnace? Achieve Faster, Cleaner, and More Efficient Metal Melting
- Why does increasing the section number of a cold crucible improve energy efficiency? Maximize Your Melting Potential



















