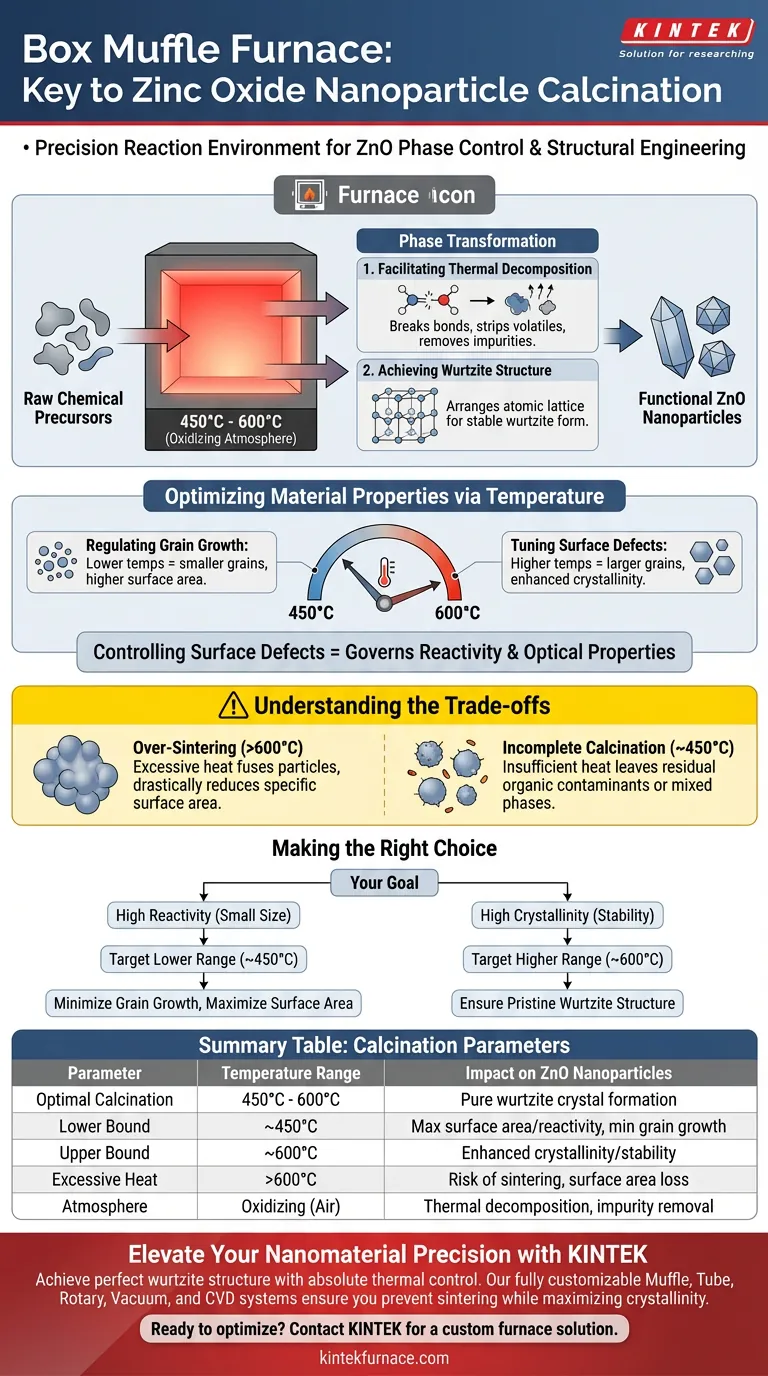
A box muffle furnace serves as the precise reaction environment required to transform raw chemical precursors into functional zinc oxide (ZnO) nanoparticles. By maintaining a stable, high-temperature oxidizing atmosphere—typically between 450°C and 600°C—the furnace facilitates the thermal decomposition and oxidation-reduction reactions necessary to convert amorphous materials into a highly crystalline wurtzite structure.
Core Takeaway The box muffle furnace is not merely a heating device; it is a tool for phase control and structural engineering. It enables the complete conversion of precursors into zinc oxide while providing the thermal precision needed to regulate grain growth and surface defects, which directly dictate the nanoparticle's final performance.

The Mechanism of Phase Transformation
Facilitating Thermal Decomposition
The primary function of the furnace is to provide enough thermal energy to break down the chemical bonds of the precursor materials. In an air atmosphere, this environment drives oxidation-reduction reactions that strip away volatile components. This step is essential for removing impurities and initiating the transition from a raw chemical mixture to a pure oxide form.
Achieving the Wurtzite Structure
Zinc oxide requires specific thermal conditions to arrange its atomic lattice correctly. The muffle furnace ensures the material reaches the thermodynamic stability required to form the wurtzite structure, which is the hexagonal crystal system most common for stable ZnO. Without this sustained high heat, the material would remain amorphous or incompletely crystallized, lacking the desired physical properties.
Optimizing Material Properties via Temperature
Regulating Grain Growth
The specific temperature setting on the furnace acts as a throttle for particle size. Operating within the typical range of 450°C to 600°C allows researchers to control how much the crystal grains grow. Higher temperatures generally promote diffusion and larger grains, while lower temperatures within the effective range help maintain smaller, finer nanostructures.
Tuning Surface Defects
The performance of zinc oxide often depends on its surface chemistry and defect density. By precisely controlling the calcination temperature, you can manipulate the concentration of these surface defects. This "tuning" capability is critical, as surface defects often govern the material's reactivity and optical characteristics.
Understanding the Trade-offs
The Risk of Over-Sintering
While high temperatures ensure high crystallinity, exceeding the optimal range can be detrimental. Excessive heat can cause the nanoparticles to fuse together (sinter), drastically reducing their specific surface area. This loss of surface area can compromise the unique advantages provided by the "nano" scale of the material.
The Danger of Incomplete Calcination
Conversely, setting the furnace temperature too low in an attempt to keep particles small carries its own risks. Insufficient heat may result in the incomplete decomposition of precursors. This leaves behind residual organic contaminants or mixed phases that degrade the purity and function of the zinc oxide.
Making the Right Choice for Your Goal
To optimize your zinc oxide nanoparticles, align your furnace settings with your specific application requirements:
- If your primary focus is High Reactivity (Small Size): Target the lower end of the calcination range (closer to 450°C) to minimize grain growth and maximize surface area.
- If your primary focus is High Crystallinity (Stability): Target the higher end of the calcination range (closer to 600°C) to ensure a pristine wurtzite structure with fewer structural faults.
By treating the box muffle furnace as a precision instrument for structural design rather than just an oven, you gain control over the fundamental physics of your nanomaterials.
Summary Table:
| Parameter | Temperature Range | Impact on ZnO Nanoparticles |
|---|---|---|
| Optimal Calcination | 450°C - 600°C | Facilitates pure wurtzite crystal structure formation |
| Lower Bound | ~450°C | Maximizes surface area and reactivity; minimizes grain growth |
| Upper Bound | ~600°C | Enhances crystallinity and thermodynamic stability |
| Excessive Heat | >600°C | Risk of sintering and significant loss of surface area |
| Atmosphere | Oxidizing (Air) | Ensures thermal decomposition and removal of volatile impurities |
Elevate Your Nanomaterial Precision with KINTEK
Achieving the perfect wurtzite structure in zinc oxide nanoparticles requires more than just heat—it requires absolute thermal control. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of material research and manufacturing.
Our furnaces are backed by expert R&D and are fully customizable to meet your unique calcination profiles, ensuring you prevent sintering while maximizing crystallinity. Whether you are a lab researcher or a high-volume manufacturer, KINTEK's high-temperature solutions provide the stability and uniformity your materials deserve.
Ready to optimize your nanoparticle synthesis? Contact KINTEK today for a custom furnace solution!
Visual Guide
References
- Kamilia Madi, Abdeltif Amrane. Green Fabrication of ZnO Nanoparticles and ZnO/rGO Nanocomposites from Algerian Date Syrup Extract: Synthesis, Characterization, and Augmented Photocatalytic Efficiency in Methylene Blue Degradation. DOI: 10.3390/catal14010062
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What role does an industrial-grade high-temperature muffle furnace play in the calcination of Barium Titanate powders?
- How is a laboratory muffle furnace used in 3D-printed PP-CF cross-linking? Achieve Thermal Stability at 150 °C
- What are the main components of a muffle furnace? Key Parts for Precision High-Temp Control
- How is a laboratory muffle furnace utilized in carbon nitride exfoliation? Optimize Thermal Processing Strategies
- What is a digital muffle furnace and why is it important? Unlock Precision Heating for Your Lab
- How does an industrial high-temperature box furnace perform solution treatment for the SS317L layer in clad plates?
- How is the temperature controlled in a muffle furnace? Master Precise Heating for Your Lab
- Why are muffle furnaces considered versatile in industrial and laboratory settings? Unlock Precision Heating for Diverse Applications



















