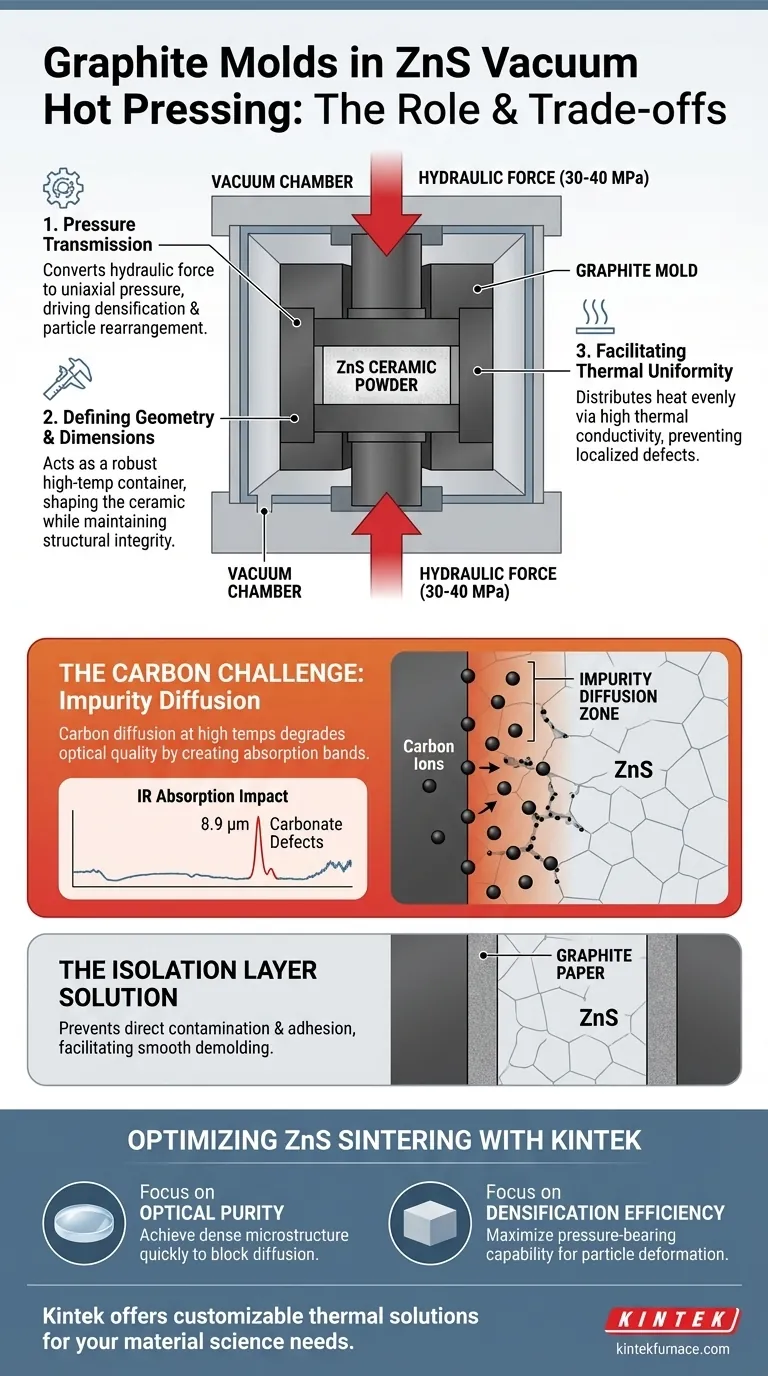
Graphite molds act as both the defining vessel and the primary engine of densification during the vacuum hot pressing of Zinc Sulfide (ZnS). They function as high-temperature containers that shape the ceramic powder while simultaneously serving as the critical medium to transmit hydraulic pressure, forcing the particle rearrangement and plastic deformation necessary to create a solid ceramic.
Core Takeaway Graphite molds are indispensable for converting external hydraulic force into the internal pressure required to sinter ZnS, but they introduce a complex chemical variable. While they facilitate physical densification through heat and pressure transfer, they also act as a carbon source that can compromise the material's optical purity through diffusion.

The Mechanics of Densification
Acting as a Pressure Transmission Medium
The most critical role of the graphite mold is to serve as a bridge between the machinery and the material.
The mold transmits mechanical force generated by the hydraulic cylinder directly to the ZnS powder. By conveying uniaxial pressures (typically ranging from 30 to 40 MPa), the mold forces the ceramic particles to rearrange and undergo plastic deformation.
Defining Geometry and Dimensions
At the most basic level, the mold acts as a robust container.
It constrains the loose ZnS powder into a specific shape and holds it there throughout the process. The mold must maintain its structural integrity and dimensional stability even while subjected to immense axial pressure and temperatures that can reach 1800°C.
Facilitating Thermal Uniformity
Beyond pressure, the mold plays a vital role in thermal regulation.
Graphite possesses excellent thermal conductivity, which helps distribute heat evenly across the ceramic sample. This ensures that the sintering process occurs uniformly throughout the volume of the material, preventing localized defects caused by uneven heating.
Understanding the Trade-offs: The Carbon Challenge
The Risk of Carbon Diffusion
While graphite is mechanically ideal, it presents a chemical challenge known as "impurity diffusion."
At high sintering temperatures, the mold acts as a carbon source. Carbon ions can detach from the mold and diffuse into the ZnS ceramic, primarily traveling along the grain boundaries of the material.
Impact on Optical Performance
The intrusion of carbon is not merely a structural issue; it degrades the optical quality of the ceramic.
When carbon ions penetrate the ZnS, they form carbonate defects that create a distinct infrared absorption band at 8.9 μm. For optical applications, this impurity significantly reduces the material's performance and transmission clarity.
The Role of Isolation Layers
To mitigate direct contamination and mechanical adhesion, operators often utilize graphite paper.
Lining the mold cavity with graphite paper acts as an isolation layer. This prevents the metal powders from reacting with or sticking to the mold walls, ensuring the final ceramic can be demolded smoothly without surface damage.
Making the Right Choice for Your Goal
The use of graphite molds requires balancing the need for mechanical pressure against the risk of chemical contamination.
- If your primary focus is Optical Purity: You must optimize the process to achieve a dense microstructure quickly, as a dense structure helps block the diffusion of carbon ions from the mold.
- If your primary focus is Densification Efficiency: Focus on the mold's ability to withstand high uniaxial pressures (up to 40 MPa) to maximize plastic deformation and particle rearrangement.
Success in ZnS sintering lies in utilizing the mold's mechanical strength while actively suppressing its chemical tendency to contaminate the grain boundaries.
Summary Table:
| Feature | Role in ZnS Sintering | Impact on Performance |
|---|---|---|
| Pressure Transmission | Converts hydraulic force to uniaxial pressure (30-40 MPa) | Drives particle rearrangement and densification. |
| Geometry Control | Provides structural containment and shaping | Ensures dimensional stability and structural integrity. |
| Thermal Conductivity | Distributes heat uniformly across the sample | Prevents localized defects through even sintering. |
| Chemical Interaction | Potential carbon source for diffusion | Can cause 8.9 μm infrared absorption defects. |
| Isolation Layer | Graphite paper lining | Prevents adhesion and facilitates smooth demolding. |
Elevate Your Material Sintering with KINTEK
Precision in ZnS sintering requires a delicate balance between mechanical force and chemical purity. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temperature furnaces designed to meet your unique materials science needs.
Whether you are aiming for peak optical clarity or maximum densification efficiency, our specialists are ready to provide the custom thermal solutions your research demands. Contact us today to optimize your sintering process!
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How does the porosity of materials differ between hot pressing and cold compacting and sintering? Compare Methods for Optimal Density
- How are hot press furnaces involved in semiconductor manufacturing? Essential for Wafer Bonding in 3D ICs
- What is the role of the vacuum environment in SiC/ZTA sintering? Enhance Densification & Material Purity
- What are the primary applications of vacuum press technology? Achieve Superior Material Bonding and Shaping
- What is the use of a hot press? Achieve Perfect Bonding & High-Performance Materials
- How does the heating mechanism of Spark Plasma Sintering (SPS) function? Enhance TiC/SiC Composite Fabrication
- In which industries is hot pressing commonly used? Essential for Aerospace, Ceramics, and Electronics
- Why is 'final short-time pressing' important in vacuum hot pressing? Unlock Maximum Material Density



















