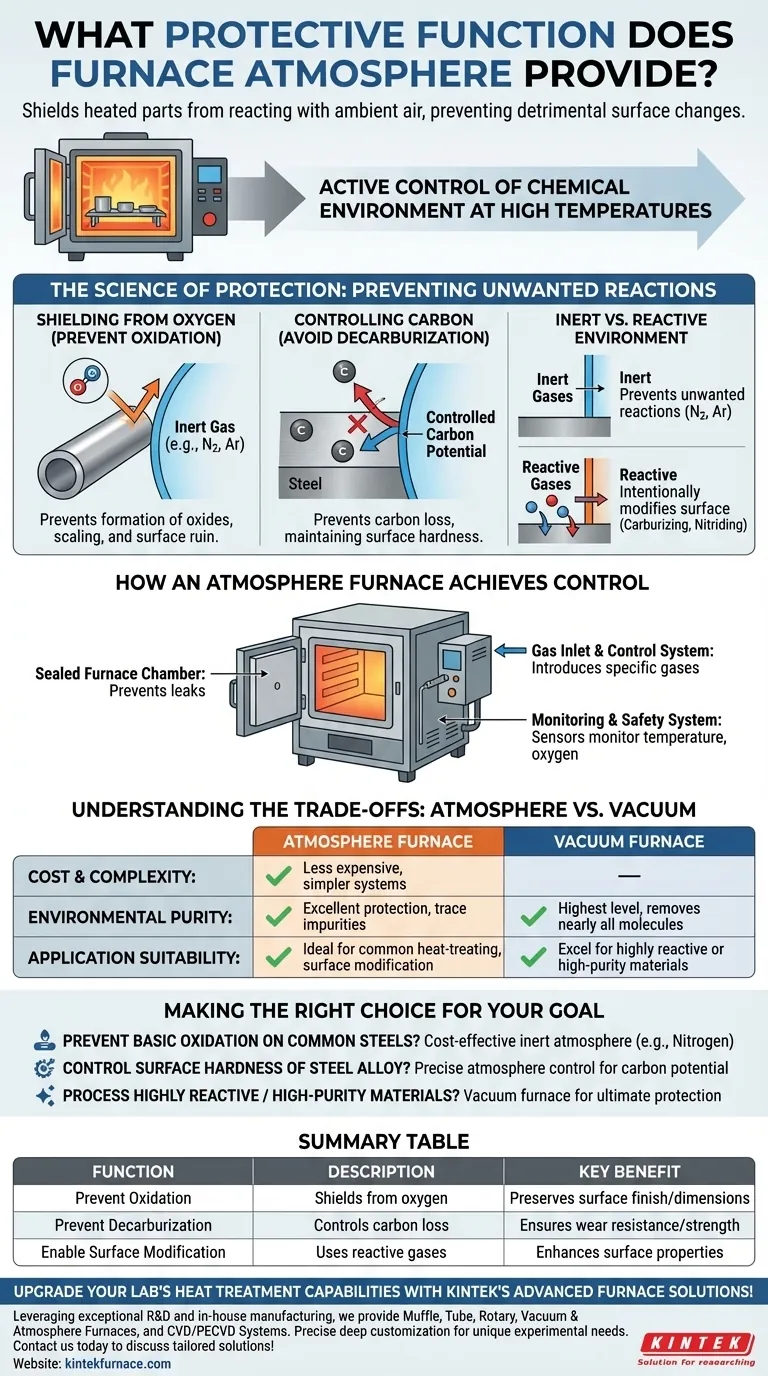
At its core, a protective furnace atmosphere creates a controlled chemical environment that shields heated parts from reacting with ambient air. This inert or reactive gas barrier is essential for preventing detrimental surface changes, such as oxidation (scaling) and decarburization (carbon loss), which can compromise the integrity and performance of the final component.
The primary function of a protective atmosphere is not merely to shield a part, but to actively control the chemical environment at high temperatures. This ensures the material's surface properties are preserved or intentionally modified to meet precise engineering specifications.

The Science of Protection: Preventing Unwanted Reactions
When metals are heated, their reactivity increases dramatically. A protective atmosphere directly counteracts the negative effects that would otherwise occur in open air, ensuring the material properties are not degraded during the thermal process.
Shielding from Oxygen to Prevent Oxidation
At high temperatures, most metals readily react with oxygen in the air to form oxides on their surface. This process, known as oxidation or scaling, can ruin surface finish, alter dimensions, and create a brittle outer layer that must be removed in a costly secondary step. A protective atmosphere displaces the oxygen, creating an inert environment where these reactions cannot occur.
Controlling Carbon to Avoid Decarburization
For carbon steels, exposure to oxygen and water vapor at high temperatures can cause carbon to diffuse out of the surface. This loss of carbon, or decarburization, results in a soft, weakened surface layer, which can be catastrophic for components that rely on surface hardness for wear resistance, such as gears or bearings. The atmosphere's composition can be controlled to have a specific "carbon potential," preventing this carbon loss.
Creating an Inert or Reactive Environment
Protective atmospheres can be either inert or reactive. Inert atmospheres, using gases like argon or nitrogen, simply prevent unwanted reactions. Reactive atmospheres, on the other hand, are designed to intentionally cause a desired surface reaction, such as carburizing (adding carbon) or nitriding (adding nitrogen) to harden the surface.
How an Atmosphere Furnace Achieves Control
A specialized furnace is required to contain and manage the protective atmosphere. This is accomplished through a combination of precise mechanical design and sophisticated control systems.
The Sealed Furnace Chamber
The process begins with a tightly sealed heating chamber. The furnace body and door are constructed with robust seals to prevent the protective gas from leaking out and, more importantly, to stop ambient air from leaking in and contaminating the controlled environment.
The Gas Inlet and Control System
Dedicated gas inlet and outlet systems allow for the introduction of specific gases—such as nitrogen, argon, or hydrogen mixtures—into the chamber. An atmosphere control system precisely regulates the flow rates and ratios of these gases to create and maintain the desired chemical environment throughout the heating cycle.
The Monitoring and Safety System
To ensure process integrity, sensors constantly monitor critical parameters like temperature and oxygen content within the furnace. If a sensor detects an anomaly—such as an oxygen leak—the system can trigger an alarm or initiate an automatic shutdown to protect both the furnace and the parts being processed.
Understanding the Trade-offs: Atmosphere vs. Vacuum
While highly effective, atmosphere-controlled furnaces are not the only solution. They exist on a spectrum of environmental control, with vacuum furnaces representing the primary alternative.
Cost and Complexity
Atmosphere furnaces are generally less expensive to purchase and operate than vacuum furnaces. The mechanical systems required to introduce and manage gases at or near atmospheric pressure are simpler than the high-power pumps and robust chambers needed to achieve a deep vacuum.
Level of Environmental Purity
A vacuum furnace provides the highest level of purity by removing nearly all molecules from the chamber, offering the ultimate protection for extremely reactive materials like titanium or refractory metals. Atmosphere furnaces provide excellent protection but will always contain trace amounts of impurities from the supply gas or minor leaks.
Application Suitability
Atmosphere furnaces are ideal for a vast range of common heat-treating processes, including neutral hardening, annealing, and carburizing of steels. Their ability to use reactive gases is a key advantage for surface modification treatments. Vacuum furnaces excel where even minimal surface interaction is unacceptable.
Making the Right Choice for Your Goal
The decision to use a specific type of protective atmosphere depends entirely on the material being treated and the desired final properties.
- If your primary focus is preventing basic oxidation on common steels: A simple inert atmosphere (like nitrogen) provides a cost-effective and highly reliable solution.
- If your primary focus is controlling the surface hardness of a steel alloy: You need a furnace with precise atmosphere control to manage the carbon potential, preventing decarburization or enabling carburization.
- If your primary focus is processing highly reactive or high-purity materials: A vacuum furnace is often the superior choice, as it eliminates nearly all potential for atmospheric contamination.
Ultimately, mastering the furnace atmosphere is fundamental to transforming a raw material into a component with predictable and reliable performance.
Summary Table:
| Function | Description | Key Benefit |
|---|---|---|
| Prevent Oxidation | Shields heated parts from oxygen to avoid surface scaling | Preserves surface finish and dimensions |
| Prevent Decarburization | Controls carbon loss in steels to maintain hardness | Ensures wear resistance and component strength |
| Enable Surface Modification | Uses reactive gases for carburizing or nitriding | Enhances surface properties for specific applications |
Upgrade your lab's heat treatment capabilities with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental needs, from preventing oxidation to enabling precise surface modifications. Contact us today to discuss how our tailored furnace atmospheres can enhance your material performance and efficiency!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance



















