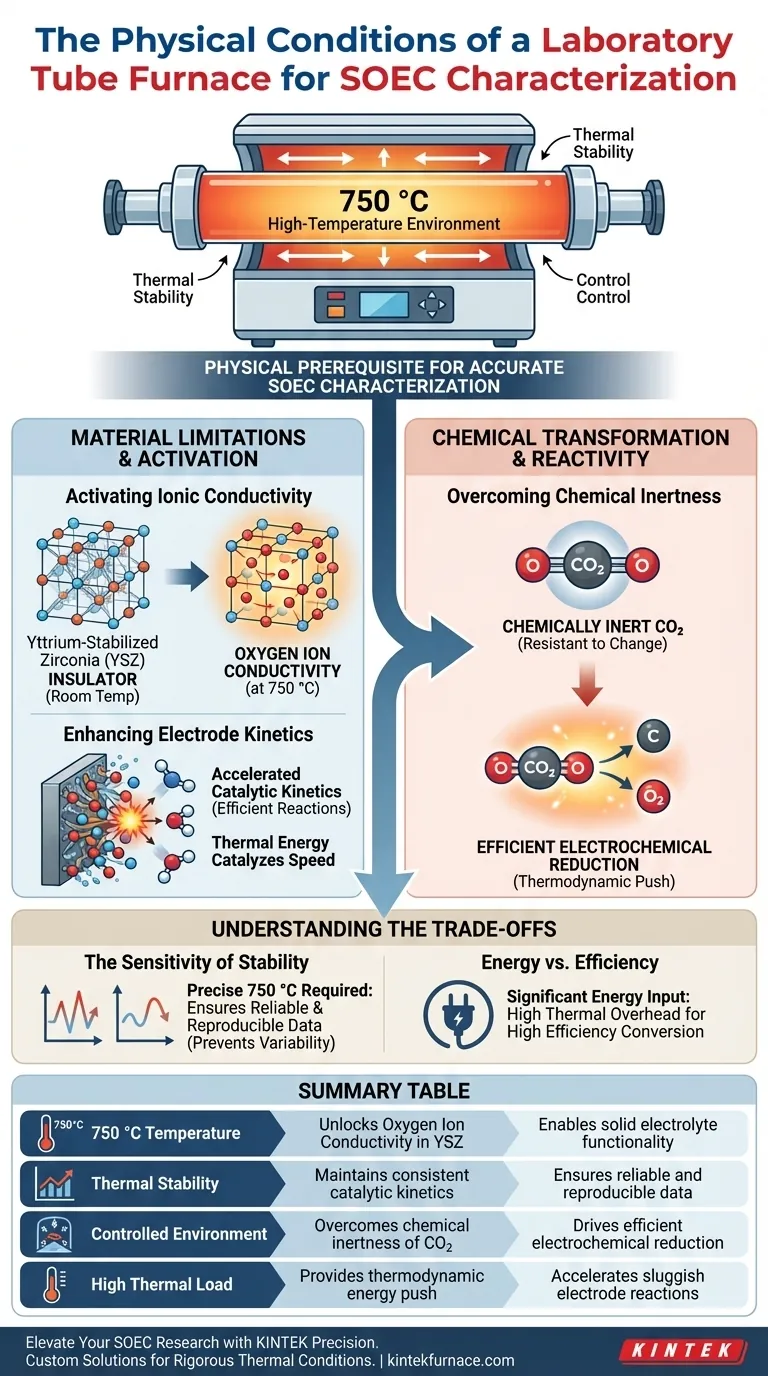
A laboratory Tube Furnace provides a stable, strictly controlled high-temperature environment, typically maintained at approximately 750 °C. This specific thermal condition is the physical prerequisite for accurately characterizing and operating Solid Oxide Electrolysis Cells (SOEC).
The furnace acts as an activation environment rather than just a heating element. Its primary function is to reach the thermal threshold necessary to unlock oxygen ion conductivity in solid electrolytes and drive the catalytic reduction of chemically inert molecules.

The Physical Necessity of High Temperature
To understand why a tube furnace is required, one must look at the material limitations of SOEC components at room temperature. The furnace creates the physical conditions needed to overcome these limitations.
Activating Ionic Conductivity
The core component of an SOEC is the electrolyte, typically made of Yttrium-Stabilized Zirconia (YSZ).
At lower temperatures, YSZ acts as an insulator. The 750 °C environment provided by the furnace is essential to ensure the material achieves sufficient oxygen ion conductivity. Without this specific thermal condition, the ions cannot move through the electrolyte, and the cell cannot function.
Enhancing Electrode Kinetics
Heat is a catalyst for speed. The high-temperature environment significantly enhances the catalytic kinetics of the electrodes.
In an SOEC, the chemical reactions at the electrode interfaces are complex. The thermal energy supplied by the furnace accelerates these reactions, ensuring the system operates efficiently rather than stalling due to sluggish reaction rates.
Enabling Chemical transformation
Beyond material properties, the physical conditions of the furnace are dictated by the difficulty of the chemical reaction being performed.
Overcoming Chemical Inertness
A primary use case for SOEC is the electrochemical reduction of carbon dioxide (CO2).
CO2 is a chemically inert molecule, meaning it is resistant to change and difficult to break apart. The 750 °C environment provides the thermodynamic push required to overcome this inertness, allowing the electrochemical reduction to proceed efficiently.
Understanding the Trade-offs
While the high temperature is necessary, the strict requirement for a "stable and controlled" environment introduces specific operational challenges.
The Sensitivity of Stability
The reference emphasizes that the environment must be stable and controlled.
If the furnace fails to maintain a precise 750 °C, the conductivity of the YSZ will fluctuate, and the catalytic kinetics will vary. This instability renders characterization data unreliable, as you cannot distinguish between cell performance and environmental fluctuation.
Energy vs. Efficiency
Operating at 750 °C requires significant energy input.
While this temperature is necessary to activate the YSZ and reduce CO2, it represents a high thermal overhead. The trade-off for high efficiency in chemical conversion is the requirement for a robust, energy-intensive thermal management system (the tube furnace).
Making the Right Choice for Your Goal
When setting up a tube furnace for SOEC characterization, focus on the specific aspect of the cell you are testing.
- If your primary focus is Electrolyte Analysis: Ensure your furnace can hold 750 °C with absolute precision to accurately measure the oxygen ion conductivity of materials like YSZ.
- If your primary focus is Carbon Capture/Conversion: Prioritize the furnace's ability to maintain high heat under load to ensure the efficient reduction of inert CO2 molecules.
Success in SOEC characterization relies entirely on maintaining a thermal environment that turns solid ceramics into ionic conductors and inert gases into reactive fuels.
Summary Table:
| Physical Condition | Role in SOEC Characterization | Key Benefit |
|---|---|---|
| 750 °C Temperature | Unlocks Oxygen Ion Conductivity in YSZ | Enables solid electrolyte functionality |
| Thermal Stability | Maintains consistent catalytic kinetics | Ensures reliable and reproducible data |
| Controlled Environment | Overcomes chemical inertness of CO2 | Drives efficient electrochemical reduction |
| High Thermal Load | Provides thermodynamic energy push | Accelerates sluggish electrode reactions |
Elevate Your SOEC Research with KINTEK Precision
Precise thermal control is the difference between breakthrough data and unreliable results. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems specifically engineered to maintain the rigorous conditions required for Solid Oxide Electrolysis Cell characterization.
Backed by expert R&D and manufacturing, our high-temperature furnaces are fully customizable to meet your unique laboratory needs—ensuring your YSZ electrolytes and CO2 reduction experiments perform at peak efficiency.
Ready to optimize your thermal environment? Contact KINTEK today for a custom solution.
Visual Guide
References
- Kristóf Stágel, Katharina Bica. Online Coupling High‐Temperature Electrolysis with Carbonylation Reactions: A Powerful Method for Continuous Carbon Dioxide Utilization. DOI: 10.1002/anie.202420578
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the industrial design advantages of using a tube furnace for ex-situ reduction of catalysts? Optimize Efficiency
- What is the function of a high-temperature tube furnace in the synthesis of SPC-Fe? Master Graphitic Carbon Production
- Why is a high-temperature tube furnace required for the annealing process during graphene growth? Optimize Substrates
- Why is a tube furnace with programmable temperature control necessary for graphene? Ensure High-Quality Graphene on Silver
- Why is the iodine source placed at the upstream end of the tube furnace? Optimizing I-NC Chemical Vapor Deposition
- What are the main features and functions of a laboratory tube furnace? Unlock Precise High-Temp Control for Your Lab
- What conditions do tube furnaces provide for Au-Seeded TiO2 nanowires? Master Precision Thermal Synthesis
- How does multi-zone heating benefit the 70mm tube furnace? Unlock Precise Thermal Control for Advanced Materials



















