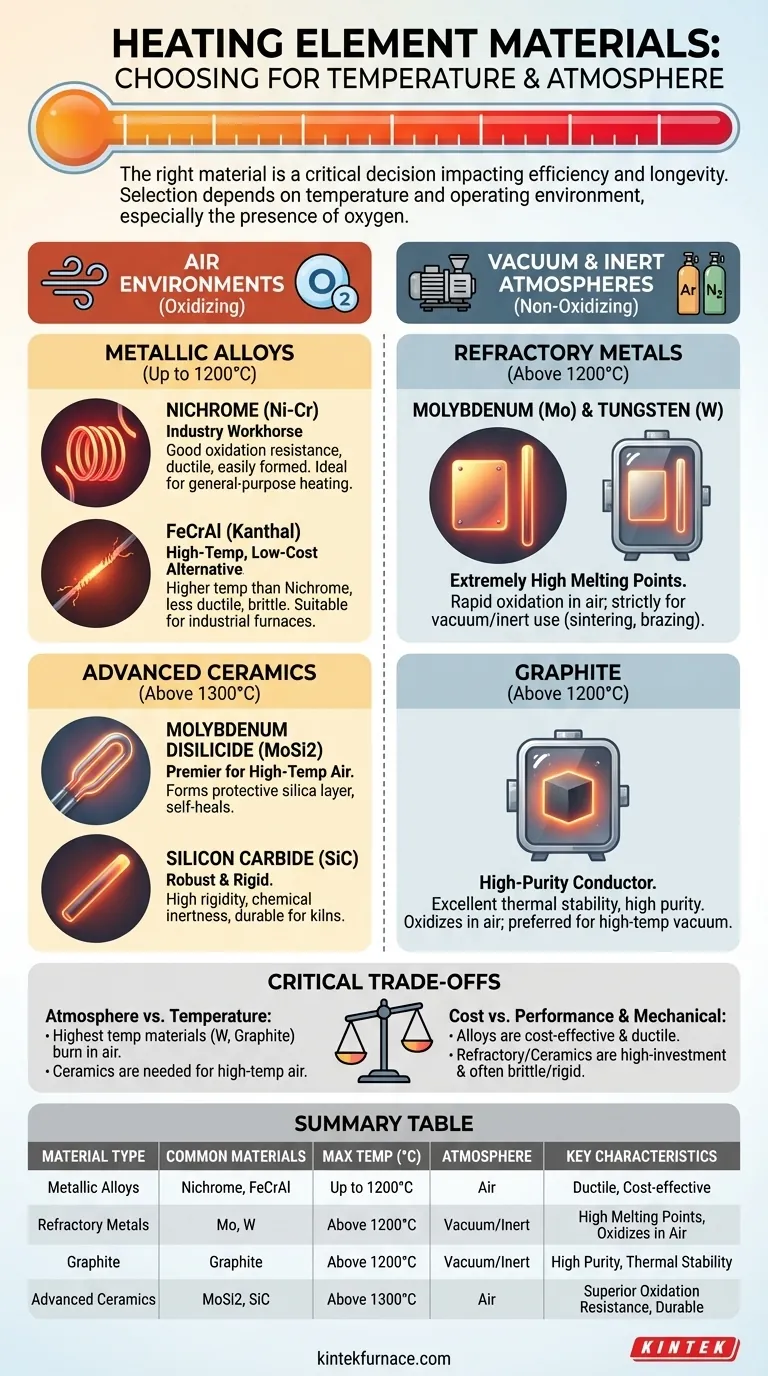
The choice of heating element material is a critical engineering decision that directly impacts process efficiency, reliability, and longevity. For lower temperature applications (typically below 1200°C) in air, nickel-chromium (Nichrome) and iron-chromium-aluminum (FeCrAl) alloys are the dominant choices. For high-temperature processes, especially in vacuum or inert atmospheres, the selection shifts to specialized materials like graphite, molybdenum, and tungsten, or advanced ceramics like Molybdenum Disilicide for use in air.
Selecting the right material is not merely about its maximum temperature rating. The most crucial factor is the operating environment—specifically, the presence of oxygen—which dictates whether a metallic alloy, a refractory metal, or an advanced ceramic is the only viable option.

The Foundation: Metallic Alloys for Air Environments
The most common heating elements are metallic alloys designed to function reliably in the presence of oxygen. They achieve this by forming a stable, protective oxide layer on their surface that prevents further degradation.
Nickel-Chromium (Nichrome): The Industry Workhorse
Nichrome, typically an alloy of 80% nickel and 20% chromium, is the most widely used heating element material. Its popularity stems from its excellent balance of properties.
It has a high melting point (~1400°C), resists oxidation effectively at high temperatures, and is highly ductile, allowing it to be easily formed into coils and complex shapes.
Iron-Chromium-Aluminum (FeCrAl): The High-Temp, Low-Cost Alternative
FeCrAl alloys, often known by the trade name Kanthal, can operate at slightly higher temperatures than Nichrome. They are a cost-effective alternative for many applications.
Their primary trade-off is lower ductility, making them more brittle and challenging to form compared to Nichrome. However, their high-temperature capability and lower cost make them ideal for industrial furnaces and appliances.
Stainless Steel: For Specialized Applications
While not a primary high-temperature element, stainless steel is used for lower-temperature processes where its specific properties are advantageous.
It is often employed in applications like aluminum brazing at higher partial pressures, where its corrosion resistance and mechanical properties are well-suited for the specific process chemistry.
Pushing the Limits: High-Temperature & Vacuum Materials
When temperatures exceed the limits of standard alloys, or when the process must occur in a controlled atmosphere, a different class of materials is required. The key distinction in this category is whether the material can tolerate oxygen.
Refractory Metals: Molybdenum (Mo) & Tungsten (W)
Molybdenum and tungsten possess extremely high melting points, making them suitable for the most demanding temperature requirements in vacuum furnaces.
Their critical weakness is rapid, catastrophic oxidation when heated in the presence of air. Therefore, their use is strictly limited to vacuum or inert gas environments for processes like sintering, metal hardening, and high-temperature brazing.
Graphite: The High-Purity Conductor
Graphite is an excellent electrical conductor with outstanding thermal stability at extreme temperatures. It is also a high-purity material, which is critical for sensitive processes.
Like refractory metals, graphite will readily oxidize (burn) in air at high temperatures. It must be used in a vacuum or inert atmosphere, where it is a preferred choice for many high-temperature vacuum furnace applications.
Advanced Ceramics: The Air-Stable Champions
Advanced ceramic materials were developed to overcome the oxidation limits of refractory metals and graphite, allowing for very high-temperature operation directly in air.
Molybdenum Disilicide (MoSi2) is a premier material for high-temperature air heating. It forms a protective layer of silica (glass) on its surface that prevents oxidation and can even "self-heal" if damaged.
Silicon Carbide (SiC) is another robust ceramic known for its high rigidity and chemical inertness. It performs reliably at high temperatures in air and is a durable choice for many furnace and kiln applications.
Understanding the Critical Trade-offs
Choosing a material involves balancing competing factors. A decision based on temperature alone will often lead to failure.
Atmosphere vs. Temperature
This is the most important trade-off. The materials capable of reaching the absolute highest temperatures (Tungsten, Graphite) will be destroyed by oxygen. If your high-temperature process must occur in air, you are limited to advanced ceramics like MoSi2 or SiC, or a high-grade FeCrAl alloy at the lower end of the "high-temp" spectrum.
Cost vs. Performance
There is a clear cost hierarchy. Nichrome and FeCrAl alloys are the most cost-effective for general use. Refractory metals and advanced ceramics represent a significant investment, justified only by the extreme temperature or specific atmospheric requirements of a specialized process.
Mechanical Properties vs. Application
Ductile materials like Nichrome are easily formed into compact coiled elements. Brittle materials like Silicon Carbide are typically supplied as rigid rods. The physical constraints of your equipment can limit your material options or dictate the element's shape (e.g., rods, bent elements, or custom panels).
Making the Right Choice for Your Application
Your final selection should be guided by your primary operational goal.
- If your primary focus is general-purpose heating in air below 1200°C: Nickel-chromium (Nichrome) offers the best overall balance of performance, ductility, and reliability.
- If your process requires very high temperatures (above 1300°C) in an oxygen-rich atmosphere: Molybdenum Disilicide (MoSi2) or Silicon Carbide (SiC) are the necessary choices due to their exceptional oxidation resistance.
- If your process involves a vacuum or inert gas environment at high temperatures: Graphite, Molybdenum, or Tungsten provide superior performance and purity for applications like sintering or specialized brazing.
- If cost is the primary constraint for a medium-to-high temperature application in air: Iron-chromium-aluminum (FeCrAl) alloys are a viable, lower-cost alternative to Nichrome.
By understanding the interplay between temperature, atmosphere, and material properties, you can select an element that ensures the efficiency and success of your thermal process.
Summary Table:
| Material Type | Common Materials | Max Temperature Range (°C) | Suitable Atmosphere | Key Characteristics |
|---|---|---|---|---|
| Metallic Alloys | Nickel-Chromium (Nichrome), Iron-Chromium-Aluminum (FeCrAl) | Up to 1200°C | Air | Good oxidation resistance, ductile, cost-effective |
| Refractory Metals | Molybdenum, Tungsten | Above 1200°C | Vacuum or Inert | High melting points, oxidizes in air |
| Graphite | Graphite | Above 1200°C | Vacuum or Inert | High purity, excellent thermal stability |
| Advanced Ceramics | Molybdenum Disilicide (MoSi2), Silicon Carbide (SiC) | Above 1300°C | Air | Superior oxidation resistance, durable |
Upgrade your laboratory's heating capabilities with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse labs with tailored options like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, boosting efficiency and reliability. Contact us today to discuss how we can optimize your thermal processes and deliver superior performance!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- What tasks does a high-temperature vacuum sintering furnace perform for PEM magnets? Achieve Peak Density
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- Why is a high vacuum essential for Ti-6Al-4V sintering? Protect Your Alloys from Embrittlement
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?



















