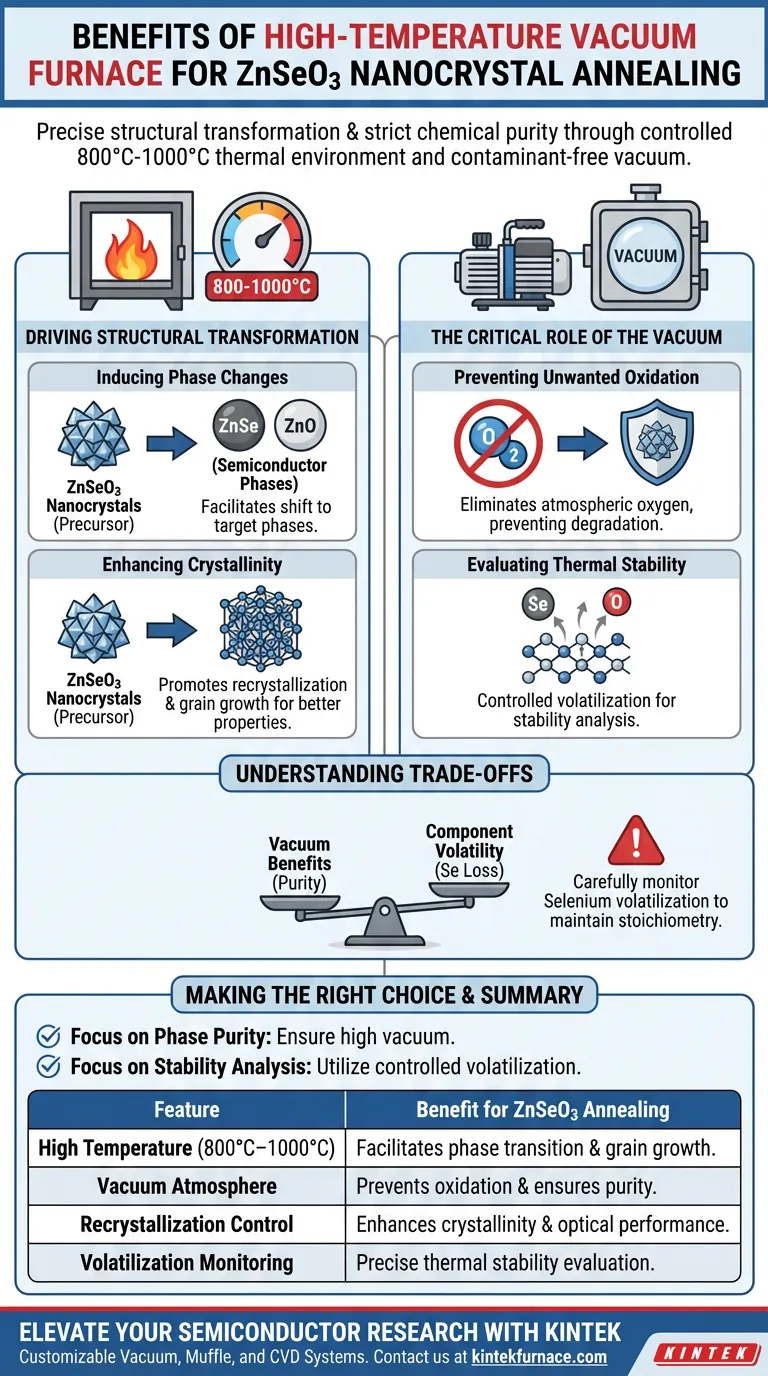
The primary benefit of using a high-temperature vacuum furnace for annealing ZnSeO3 nanocrystals is the ability to drive precise structural transformation while strictly maintaining chemical purity. This equipment facilitates the conversion of ZnSeO3 into target phases like ZnSe or ZnO by providing a thermal environment of 800°C to 1000°C, while the vacuum atmosphere prevents uncontrolled oxidation.
By combining high thermal kinetic energy with a contaminant-free vacuum environment, this process enables the controlled recrystallization of ZnSeO3 into stable semiconductor phases, serving as a critical step for tuning optical performance and evaluating thermal stability.
Driving Structural Transformation
Inducing Phase Changes
To convert ZnSeO3 nanocrystals into useful semiconductor materials, significant thermal energy is required.
A high-temperature furnace operates effectively at 800°C or 1000°C, providing the necessary heat to facilitate phase transitions. This thermal input drives the structural shift from ZnSeO3 into distinct phases such as ZnSe or ZnO.
Enhancing Crystallinity
Beyond simple phase changes, the quality of the internal crystal structure is paramount.
The thermal kinetic energy provided during the annealing process promotes recrystallization and grain growth. This significantly improves the overall crystallinity of the deposited materials, which is essential for optimizing their physical and optical properties.
The Critical Role of the Vacuum
Preventing Unwanted Oxidation
The most significant risk during high-temperature annealing is the chemical reaction of the sample with the atmosphere.
A vacuum environment is critical because it prevents unwanted oxidation during the heating process. Without a vacuum, the high temperatures required for annealing would likely degrade the nanocrystals through uncontrolled reactions with atmospheric oxygen.
Evaluating Thermal Stability
The vacuum setting serves a dual purpose: protection and evaluation.
It allows for the controlled volatilization of specific components, such as oxygen or selenium. By observing how these elements volatilize under vacuum conditions, researchers can effectively evaluate the thermal stability of the material composition.
Understanding the Trade-offs
Managing Component Volatility
While the vacuum prevents oxidation, it also lowers the boiling point of volatile elements.
You must carefully monitor the volatilization of Selenium (Se) during the process. While controlled loss is useful for stability testing, excessive volatilization can unintentionally alter the stoichiometry of the final ZnSe or ZnO phase, potentially degrading the material's intended semiconductor properties.
Making the Right Choice for Your Goal
To maximize the benefits of vacuum annealing for your specific application, align your process parameters with your desired outcome:
- If your primary focus is Phase Purity: Ensure the vacuum level is sufficiently high to eliminate all traces of atmospheric oxygen, preventing surface contamination of the ZnSe or ZnO phases.
- If your primary focus is Stability Analysis: Utilize the controlled volatilization feature to precisely measure the temperature points at which Oxygen or Selenium begin to dissociate from the lattice.
High-temperature vacuum annealing is the definitive method for converting precursor nanocrystals into high-quality semiconductors without compromising their chemical integrity.
Summary Table:
| Feature | Benefit for ZnSeO3 Annealing |
|---|---|
| High Temperature (800°C–1000°C) | Facilitates phase transition into ZnSe/ZnO and promotes grain growth. |
| Vacuum Atmosphere | Prevents uncontrolled oxidation and ensures high chemical purity. |
| Recrystallization Control | Enhances semiconductor crystallinity and tunes optical performance. |
| Volatilization Monitoring | Allows for precise thermal stability evaluation of Se and O components. |
Elevate Your Semiconductor Research with KINTEK
Precision matters when transforming ZnSeO3 nanocrystals into high-performance semiconductor phases. KINTEK provides state-of-the-art Vacuum, Muffle, and CVD systems designed to deliver the rigorous thermal stability and atmospheric control your research demands. Backed by expert R&D and manufacturing, our lab high-temperature furnaces are fully customizable to meet your unique materials science requirements.
Ready to optimize your annealing process? Contact KINTEK today to discuss our customizable furnace solutions and discover how our expertise can drive your innovation forward.
Visual Guide
References
- Gulnara Aralbayeva, А. Аkilbekov. The Thermal Stability and Photoluminescence of ZnSeO3 Nanocrystals Chemically Synthesized into SiO2/Si Track Templates. DOI: 10.3390/cryst14080730
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Why are the Sn-flux method and vacuum sealing necessary for the synthesis of NdMn2Ge2 single crystal materials?
- How are vacuum furnaces applied in the semiconductor industry? Essential for High-Purity Chip Manufacturing
- How does an industrial vacuum sintering furnace contribute to the densification of NdFeB magnets? | Expert Insights
- What is the first step in the vacuum sintering process? Master the Key to High-Performance Parts
- How does a vacuum furnace contribute to the refining and degassing of alloy melts? Enhancing Metal Purity and Density
- What role do vacuum furnaces play in electronic component manufacturing? Essential for Purity and Precision
- What cooling gases are recommended for different materials in vacuum heat treatment? Optimize Your Quenching Process
- Why is the development of high-temperature vacuum equipment and processes increasingly important? Unlock Purity and Performance in Materials



















