For vacuum heat treatment, the choice of cooling gas is dictated by the material's chemical reactivity at high temperatures. The correct gas ensures rapid cooling to achieve the desired metallurgical structure without causing harmful surface reactions. For most steels, high-purity nitrogen is the standard, while reactive metals like titanium alloys mandate the use of a truly inert gas like argon to prevent embrittlement.
The central challenge in vacuum quenching is balancing cooling speed with chemical inertness. Your gas selection must rapidly extract heat to achieve the required hardness and microstructure, while simultaneously being non-reactive with the specific alloy being treated at elevated temperatures.

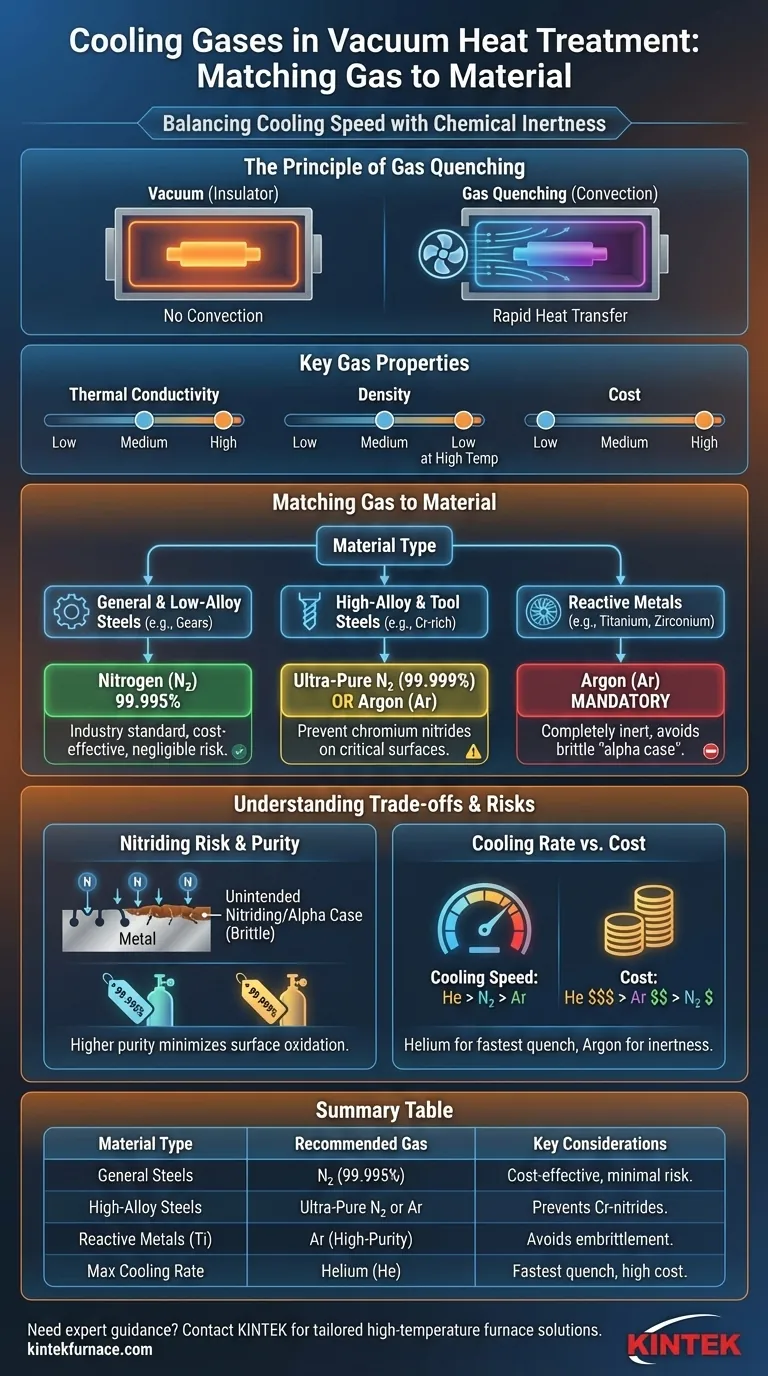
The Principle of Gas Quenching
Why a Gas is Necessary
A common misconception is that the vacuum itself provides cooling. In reality, a vacuum is an excellent insulator, making heat transfer by convection impossible and radiation inefficient at lower temperatures.
To achieve the rapid cooling (quenching) required to form specific microstructures like martensite in steel, an inert or non-reactive gas is introduced at high pressure (typically 2 to 20 bar). A powerful fan circulates this gas, enabling rapid and uniform convective heat transfer from the hot components.
Key Properties of Quench Gases
The choice between gases comes down to a few key properties:
- Thermal Conductivity: How effectively a gas can absorb and transfer heat. Helium is the most conductive, followed by nitrogen, with argon being the least conductive of the common options.
- Density: Denser gases like argon require more fan power to circulate at the same velocity compared to lighter gases like nitrogen or helium.
- Chemical Reactivity: This is the most critical factor. While nitrogen is largely inert, it can react with certain elements at high temperatures. Argon and helium are truly inert and will not react with any material.
- Cost: Nitrogen is the most economical, argon is moderately expensive, and helium is a premium, high-cost gas.
Matching the Gas to the Material
For General and Low-Alloy Steels
Nitrogen (N₂) of 99.995% purity or higher is the industry standard for these materials.
It provides a good balance of cooling performance and cost-effectiveness. For the vast majority of steels, the risk of forming undesirable nitrides during the rapid cooling phase is negligible.
For High-Alloy and Tool Steels
For steels with high concentrations of alloying elements like chromium (Cr), the choice becomes more nuanced.
These elements can have a high affinity for nitrogen, potentially forming chromium nitrides on the surface. To mitigate this, either ultra-high purity nitrogen (99.999%) is used to reduce impurities, or a switch is made to argon (Ar) for mission-critical components where no surface reaction is tolerable.
For Reactive and Non-Ferrous Metals
This category includes materials like titanium (Ti), zirconium (Zr), and certain high-aluminum alloys.
Using nitrogen for these materials is strictly prohibited. Titanium, for example, will readily react with nitrogen at quenching temperatures to form a hard, brittle surface layer known as "alpha case," which severely degrades the component's mechanical properties, especially fatigue life.
For these reactive metals, high-purity argon (Ar) is mandatory as it is completely inert.
Understanding the Trade-offs and Risks
The Danger of Unintended Nitriding
The primary risk of using the wrong gas is nitriding. This occurs when nitrogen atoms from the quenching gas diffuse into the surface of the metal, forming hard, brittle nitride compounds.
This unintended surface case can lead to premature cracks, reduced ductility, and catastrophic failure under load. This is precisely why argon is essential for reactive metals.
Cooling Rate vs. Cost
Your choice directly impacts cooling rates. With all other factors (pressure, fan speed) being equal, helium provides the fastest quench, followed by nitrogen, and then argon.
However, this performance comes at a price. The extreme cost of helium reserves it for applications where maximum cooling speed is the only priority, such as quenching extremely large cross-sections. Nitrogen offers the best all-around value, while argon is chosen for its inertness, accepting a slightly slower cooling rate as the trade-off.
The Role of Gas Purity
The purity percentage (e.g., 99.995%) is critical because it defines the level of impurities like oxygen (O₂) and water vapor (H₂O).
Even tiny amounts of these impurities can cause surface oxidation on the hot parts, compromising surface finish and integrity. Using a higher-purity gas minimizes this risk and ensures a clean, bright finish, which is one of the primary benefits of vacuum heat treatment.
Making the Right Choice for Your Process
Your decision should be based on the metallurgical requirements of the material you are processing.
- If your primary focus is cost-effective treatment of general steels: High-purity nitrogen (99.995%) is the industry standard, offering the best balance of performance and cost.
- If you are treating high-alloy or sensitive tool steels: Use ultra-high purity nitrogen (99.999%) or switch to argon to eliminate any risk of nitride formation on critical surfaces.
- If you are processing reactive materials like titanium or zirconium: You must use high-purity argon to prevent catastrophic surface embrittlement.
- If achieving the absolute maximum cooling rate is non-negotiable: High-pressure helium is the most effective quenching gas, but its high cost must be justified by the application's demands.
Choosing the correct quenching gas is fundamental to ensuring the metallurgical integrity and performance of the final component.
Summary Table:
| Material Type | Recommended Gas | Key Considerations |
|---|---|---|
| General and Low-Alloy Steels | High-Purity Nitrogen (99.995%) | Cost-effective, minimal nitride risk |
| High-Alloy and Tool Steels | Ultra-High Purity Nitrogen (99.999%) or Argon | Prevents chromium nitride formation |
| Reactive Metals (e.g., Titanium) | High-Purity Argon | Avoids embrittlement from alpha case |
| Maximum Cooling Rate Applications | Helium | Fastest quench, high cost |
Need expert guidance on selecting the right cooling gas for your vacuum heat treatment? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we can precisely meet your unique experimental requirements, helping you achieve optimal metallurgical outcomes and prevent costly errors. Contact us today to discuss how our tailored solutions can enhance your lab's efficiency and results!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What are the general operational features of a vacuum furnace? Achieve Superior Material Purity & Precision
- What role does a high-temperature vacuum heat treatment furnace play in LP-DED? Optimize Alloy Integrity Today
- What is the vacuum heat treatment process? Achieve Superior Surface Quality and Material Performance
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- What are the proper procedures for handling the furnace door and samples in a vacuum furnace? Ensure Process Integrity & Safety



















