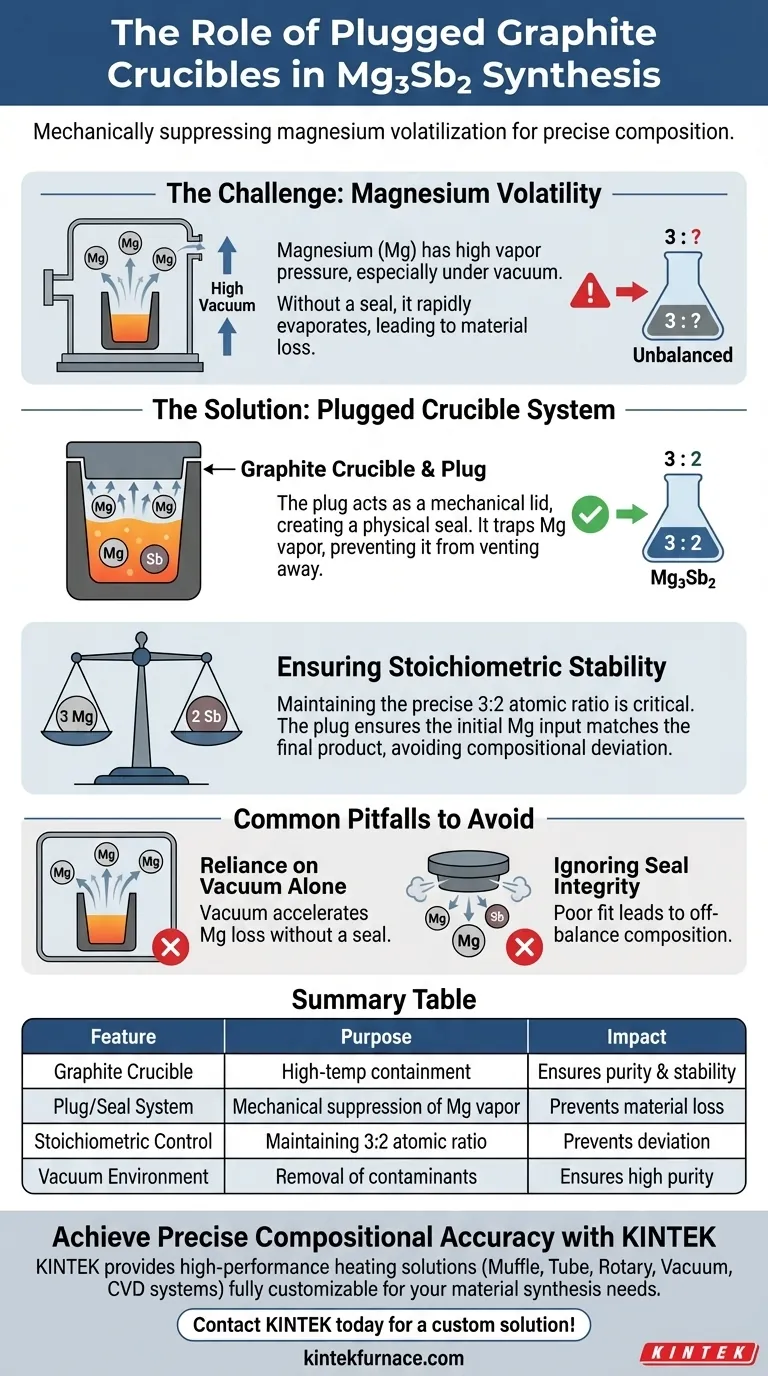
The specific purpose of using a graphite crucible equipped with a plug during the melting process of Mg3Sb2 is to mechanically suppress the volatilization of magnesium (Mg). Because magnesium has a naturally high vapor pressure, specifically under high-vacuum conditions, a physical seal is required to prevent the element from evaporating out of the melt. This containment ensures the final material retains the correct chemical ratio of components.
Core Takeaway: Magnesium is highly volatile and prone to rapid evaporation when melted in a vacuum. The plug creates a physical barrier that traps Mg vapor, maintaining the precise stoichiometric balance required to successfully synthesize Mg3Sb2.

The Challenge of Magnesium Volatility
Understanding High Vapor Pressure
Magnesium behaves differently than many other metals during thermal processing due to its high vapor pressure. When heated to the temperatures required for melting, magnesium atoms readily escape the liquid phase and turn into gas.
The Role of High Vacuum
The synthesis of Mg3Sb2 frequently takes place in high-vacuum environments to ensure purity. While the vacuum removes contaminants, it also lowers the boiling point of the magnesium.
Without a physical barrier, the vacuum environment would accelerate the evaporation of magnesium, stripping it from the crucible before the reaction is complete.
The Function of the Plug System
Creating a Physical Seal
The plug serves as a mechanical lid that seals the graphite crucible. By physically closing off the top of the container, the plug restricts the flow of gas leaving the melt.
Preventing Material Loss
This seal creates a confined space where magnesium vapor is trapped rather than vented away. This saturation helps suppress further evaporation from the liquid melt below.
Ensuring Stoichiometric Stability
To create Mg3Sb2, you must maintain a precise ratio of three magnesium atoms to two antimony atoms. If magnesium is lost to evaporation, the final compound will suffer from "compositional deviation."
The plug ensures that the amount of magnesium put into the process matches the amount in the final product.
Common Pitfalls to Avoid
Reliance on Vacuum Alone
A common misconception is that a high-vacuum chamber is sufficient for synthesis. However, for volatile elements like magnesium, a vacuum alone effectively sucks the material out of the crucible.
Ignoring Seal Integrity
The effectiveness of this method relies entirely on the quality of the physical seal provided by the plug. If the plug fits poorly, magnesium vapor will leak, leading to an off-balance chemical composition in the final ingot.
Ensuring Material Quality
If your primary focus is Compositional Accuracy:
- Ensure the plug provides a tight, consistent seal to prevent the escape of magnesium vapor.
If your primary focus is Process Consistency:
- Recognize that the plug is a critical control variable; missing or ill-fitting plugs will result in batch-to-batch variations.
By utilizing a sealed plug system, you convert a volatile, unpredictable process into a controlled synthesis that yields high-quality Mg3Sb2.
Summary Table:
| Feature | Purpose in Mg3Sb2 Melting | Impact on Final Product |
|---|---|---|
| Graphite Crucible | High-temperature containment | Ensures purity and thermal stability |
| Plug/Seal System | Mechanical suppression of Mg vapor | Prevents material loss and evaporation |
| Stoichiometric Control | Maintaining 3:2 atomic ratio | Prevents compositional deviation |
| Vacuum Environment | Removal of contaminants | Ensures high-purity material synthesis |
Achieve Precise Compositional Accuracy with KINTEK
Don’t let volatile elements compromise your research. KINTEK provides high-performance heating solutions designed for the most demanding material syntheses. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory needs. Whether you are processing magnesium alloys or advanced semiconductors, our equipment ensures the thermal stability and atmospheric control you require.
Ready to elevate your material quality? Contact KINTEK today for a custom solution!
Visual Guide
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the general ambient temperature limit for water circulating vacuum pumps? Ensure Peak Performance and Avoid Damage
- What is the function of the circulating water cooling system? Optimize Pyrolysis Oil Condensation and Yield
- What is the necessity of a cylindrical condenser in a microwave-assisted metal reduction system? Key Protection Insights
- Why is a graphite crucible selected as the high-temperature reaction vessel? Optimize Sodium-Ion Battery Synthesis
- What are the benefits of a vacuum chamber? Achieve Unmatched Process Control and Purity
- What are the limitations of ultra-pure alumina porcelain tubes? Manage Brittleness for Reliable High-Temp Use
- Why are vacuum filtration devices and specific cellulose filter papers used in hydrothermal synthesis recovery?
- What is the function of the laboratory furnace? Master Material Transformation with Precision Heating


















