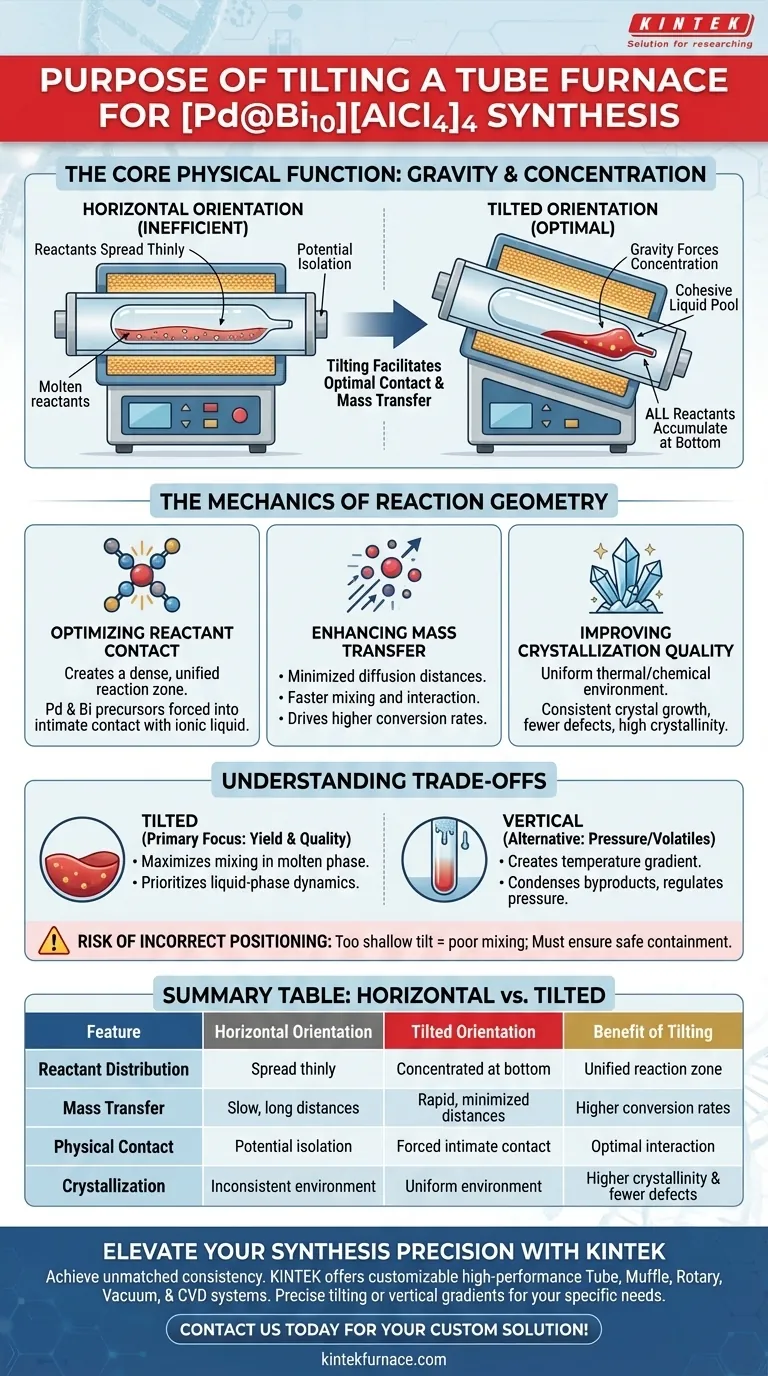
Tilting the tube resistance furnace serves a critical physical function: it utilizes gravity to force all reactants—specifically PdCl2, Bi, BiCl3, and ionic liquid precursors—to accumulate at the bottom of the ampoule once they reach a molten state. This concentration of material prevents the reactants from spreading thinly along the length of the tube, ensuring they remain in a cohesive liquid pool.
By gathering the molten reactants into a single, concentrated volume, the tilt facilitates optimal physical contact and mass transfer within the ionic liquid. This directly drives higher conversion rates and superior crystallization quality in the final cluster product.

The Mechanics of Reaction Geometry
Optimizing Reactant Contact
In the synthesis of [Pd@Bi10][AlCl4]4 clusters, the reaction environment is multiphase, involving solids transforming into a molten ionic liquid.
If the furnace were perfectly horizontal, the liquefied reactants would spread out across the bottom surface of the ampoule.
This increases the surface area but drastically reduces the depth of the liquid, potentially isolating reactants from one another.
By introducing a slight tilt, you ensure that all components slide to the lowest point of the ampoule.
This creates a dense, unified reaction zone where the Palladium and Bismuth precursors are forced into intimate contact with the ionic liquid solvent.
Enhancing Mass Transfer
Chemical synthesis in ionic liquids relies heavily on mass transfer—the movement of molecules within the fluid to react with one another.
When the reactants are pooled at the bottom due to the tilt, the diffusion distances between reacting species are minimized.
This proximity allows for more efficient mixing and interaction compared to a thin, elongated film of liquid.
Consequently, the reaction proceeds more vigorously, leading to a higher conversion rate of the raw materials into the desired cluster.
Improving Crystallization Quality
The formation of high-quality crystals requires a stable and uniform environment where nucleation can occur without interruption.
A pooled reaction mixture creates a uniform thermal and chemical environment.
This homogeneity ensures that the crystal growth is consistent, reducing defects and promoting the formation of single-phase, high-crystallinity products.
Understanding the Trade-offs
Tilt vs. Vertical Alignment
While tilting is ideal for mixing molten phases, it is important to understand how it contrasts with other configurations discussed in high-temperature synthesis.
A vertical alignment is often used to create a distinct temperature gradient.
In vertical setups, the top of the tube is kept cool to allow volatile byproducts to condense, effectively regulating internal pressure and preventing explosions.
The Risk of Incorrect Positioning
The tilted configuration prioritizes the liquid-phase reaction dynamics over the gas-phase condensation management found in vertical setups.
However, if the tilt is too shallow, you risk the "horizontal problem" of poor mixing.
Conversely, the tilt must still allow for the safe containment of the melt without compromising the structural integrity of the ampoule or the furnace's heating uniformity.
Making the Right Choice for Your Goal
To ensure the successful synthesis of [Pd@Bi10][AlCl4]4 clusters, you must align your furnace configuration with your specific process requirements.
- If your primary focus is maximizing yield and crystal quality: Ensure a slight tilt to concentrate the molten ionic liquid and precursors at the bottom of the ampoule, facilitating thorough mixing.
- If your primary focus is managing high internal pressure or volatiles: Consider how a vertical orientation (or a distinct cold zone in your tilted setup) can help condense byproducts and regulate pressure.
Ultimately, the tilt is a simple but vital geometric optimization that transforms a dispersed mixture into a highly reactive, cohesive system.
Summary Table:
| Feature | Horizontal Orientation | Tilted Orientation | Benefit of Tilting |
|---|---|---|---|
| Reactant Distribution | Spread thinly along tube length | Concentrated at the ampoule bottom | Ensures a dense, unified reaction zone |
| Mass Transfer | Slow (long diffusion distances) | Rapid (minimized distances) | Drives higher conversion rates |
| Physical Contact | Potential isolation of precursors | Forced intimate contact | Optimal interaction in ionic liquid |
| Crystallization | Inconsistent thermal environment | Uniform thermal/chemical environment | Higher crystallinity & fewer defects |
Elevate Your Synthesis Precision with KINTEK
Achieve unmatched consistency in your chemical research. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific geometry and temperature requirements. Whether you need precise tilting mechanisms for cluster synthesis or vertical gradients for volatile management, our lab high-temp furnaces provide the thermal stability your work demands.
Ready to optimize your lab's performance? Contact us today to find your custom solution!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What are the configuration options available for split tube furnaces? Customize for Precise Thermal and Atmospheric Control
- What role does a tube furnace play in the preparation of precursors? Optimize g-C3N4 Calcination Today
- What is the primary function of a tube furnace in materials science and engineering? Unlock Precise High-Temperature Processing
- How does an alumina-lined vertical tube furnace provide a stable environment for corrosion experiments? Get Expert Data
- What are some thermal processes that tube furnaces are used for? Achieve Precise Heat Treatment with Uniformity
- What metallurgical processes are performed in horizontal furnaces? Unlock Precision Heat Treatment and Sintering
- How does a tube furnace facilitate the conversion of ZIF67/MXene into CoS@C/MXene? Mastering Thermal Synthesis
- What technical advantages do three-zone tube furnaces offer? Superior Temperature Control and Flexibility



















