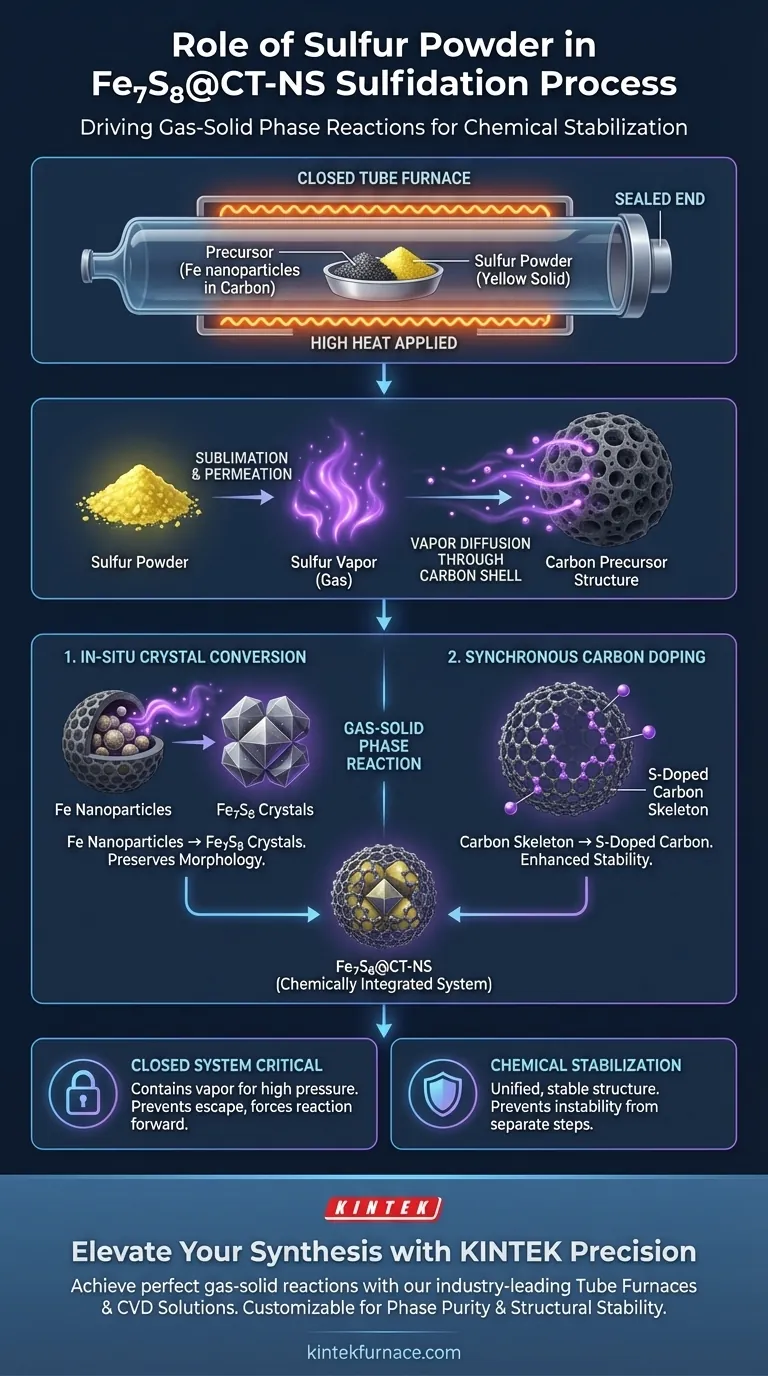
The mixing of precursor and sulfur powder serves as the catalyst for a critical gas-solid phase reaction. Upon heating within the closed environment of a tube furnace, the solid sulfur sublimates into a vapor. This vapor permeates the precursor's carbon structure to drive both the chemical conversion of the metal core and the modification of the carbon shell.
By utilizing sulfur vapor rather than a solid-solid interface, this method achieves two simultaneous outcomes: the in-situ transformation of iron nanoparticles into Fe7S8 crystals and the synchronous sulfur doping of the carbon skeleton, resulting in a chemically stable composite.

The Mechanism of Vapor Diffusion
Sublimation and Permeation
The process begins when thermal energy causes the sulfur powder to sublimate—transitioning directly from a solid to a gas.
Because the reaction occurs in a closed tube furnace, this sulfur vapor is contained and concentrated.
The vapor possesses high mobility, allowing it to permeate through the polydopamine-derived carbon layer of the precursor material to reach the internal components.
The Gas-Solid Phase Reaction
The interaction is defined as a gas-solid phase reaction.
Unlike mixing two solids which requires direct contact points, the sulfur vapor surrounds and infiltrates the solid precursor.
This ensures uniform exposure of the precursor's internal structure to the reactive sulfur species.
Simultaneous Chemical Transformations
In-Situ Crystal Conversion
Once the sulfur vapor penetrates the carbon nanotubes, it reacts with the iron-based nanoparticles housed inside.
This reaction triggers an in-situ conversion, transforming the iron nanoparticles into specific Fe7S8 crystals.
The "in-situ" nature of this process means the conversion happens within the protective carbon structure, preserving the morphology of the material.
Synchronous Carbon Doping
Simultaneously, the sulfur vapor interacts with the carbon material itself.
As the iron converts, the carbon skeleton undergoes sulfur doping, where sulfur atoms are incorporated into the carbon lattice.
This synchronous activity ensures that the final material is not just a physical mixture, but a chemically integrated system.
Understanding the Process Constraints
The Necessity of a Closed System
The reference highlights that this is a closed thermal treatment.
If the system were open, the sublimated sulfur vapor would escape rather than permeating the precursor.
The containment of the vapor is the critical variable that forces the reaction forward.
Chemical Stabilization
The ultimate goal of this specific process is chemical stabilization.
By combining the conversion of the metal and the doping of the carbon into a single step, the resulting Fe7S8@CT-NS material achieves a stable, unified structure.
Separating these steps could lead to instability or incomplete integration of the sulfur into the carbon framework.
Key Considerations for Synthesis
To maximize the effectiveness of this sulfidation process, consider your specific material goals:
- If your primary focus is Phase Purity: Ensure the tube furnace remains strictly closed to maintain the high sulfur vapor pressure required for complete in-situ conversion of the iron nanoparticles.
- If your primary focus is Structural Stability: Rely on the synchronous doping mechanism to reinforce the carbon skeleton, ensuring it chemically bonds with the sulfur rather than just coating it.
The power of this method lies in its efficiency: it leverages the natural sublimation of sulfur to perform complex internal chemistry without requiring multiple processing steps.
Summary Table:
| Process Component | Role & Mechanism | Key Outcome |
|---|---|---|
| Sulfur Powder | Sublimates into vapor at high heat | Acts as a high-mobility reactant |
| Vapor Diffusion | Permeates carbon layers | Enables gas-solid phase reaction |
| Metal Conversion | In-situ transformation of Fe | Formation of stable Fe7S8 crystals |
| Carbon Skeleton | Synchronous sulfur doping | Enhanced chemical & structural stability |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect gas-solid phase reaction requires precise thermal control and a reliable closed-system environment. KINTEK provides industry-leading Tube Furnaces, Vacuum systems, and CVD solutions designed specifically for complex processes like sulfidation and in-situ chemical transformations.
Whether you are focusing on phase purity or structural stability, our equipment is backed by expert R&D and is fully customizable to meet your unique lab requirements. Don't settle for inconsistent results—partner with the high-temperature furnace experts.
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- Xingyun Zhao, Tiehua Ma. Fe<sub>7</sub>S<sub>8</sub> Nanoparticles Embedded in Sulfur–Nitrogen Codoped Carbon Nanotubes: A High‐Performance Anode Material for Lithium‐Ion Batteries with Multilevel Confinement Structure. DOI: 10.1002/celc.202500066
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is high-vacuum encapsulation in quartz tubes required? Ensure Precision for Sn-Ag-Bi-Se-Te Composites
- Why is a tube annealing furnace used for SiC hydrogenation? Unlock Pure Atomic Surfaces for Superior Crystal Bonding
- How does the diversification of vacuum tube furnaces impact the market? Unlock Specialized Solutions for Advanced Materials
- How is a vertical alumina tube resistance furnace applied in the hydrogen reduction of bauxite residue particles?
- What are the disadvantages of tube furnace cracking when processing heavy raw materials? Avoid Costly Downtime and Inefficiency
- What are some advanced features of more elaborate tube furnaces? Unlock Precision Control for High-Temp Processes
- Why are high-temperature tube furnaces used for TiZrMoSn0.8Hf0.2 alloys? Essential Benefits for Material Science
- How do multi zone tube furnaces contribute to materials science research? Unlock Precise Temperature Control for Advanced Synthesis



















