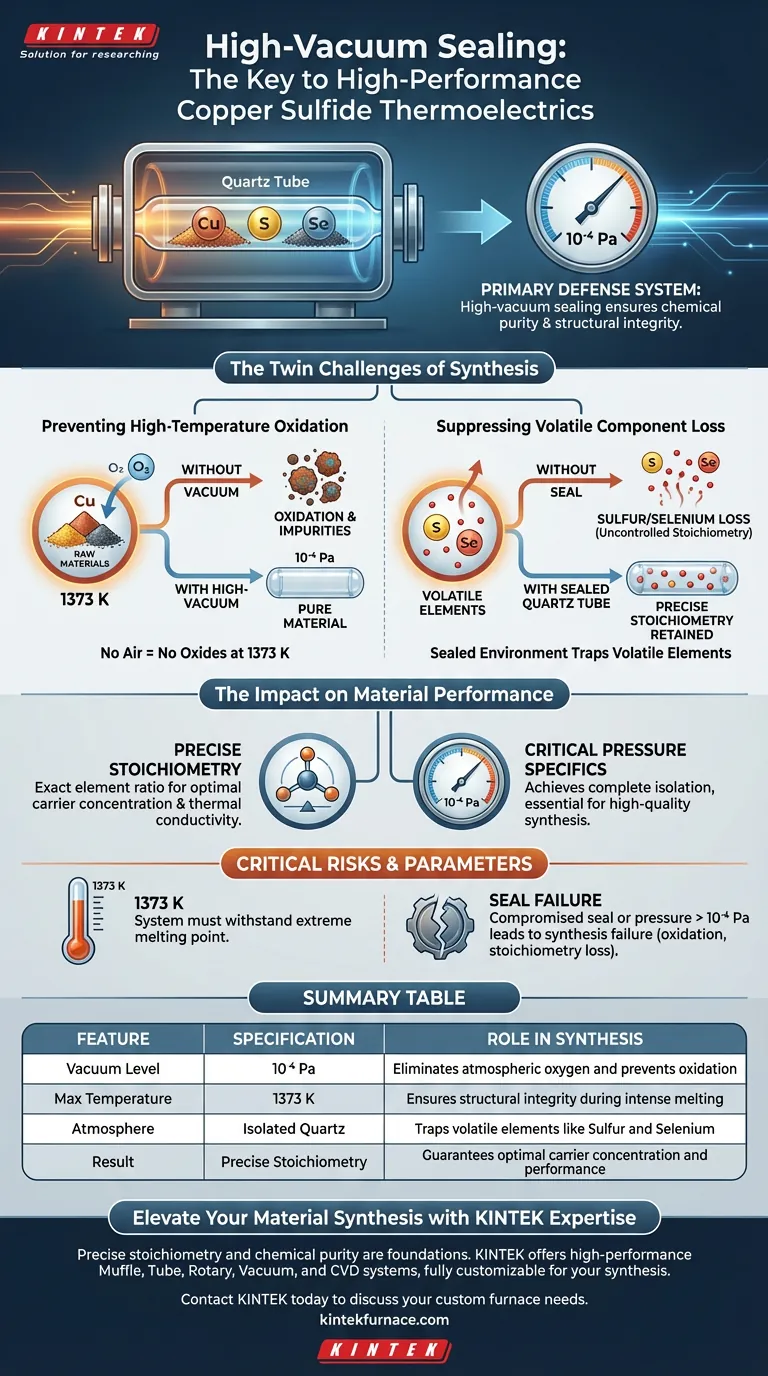
High-vacuum sealing technology acts as the primary defense system in the synthesis of copper sulfide thermoelectric materials, ensuring chemical purity and structural integrity. By evacuating quartz tubes to a specific high vacuum of 10⁻⁴ Pa prior to sealing, the process completely isolates raw materials from the external atmosphere.
The synthesis of high-performance thermoelectrics involves extreme heat that typically degrades reactive components. High-vacuum sealing solves this by creating a closed environment that prevents oxidation and traps volatile elements, ensuring the final material retains the precise chemical ratios required for optimal performance.
The Twin Challenges of Synthesis
Creating high-performance thermoelectric materials requires subjecting raw elements to intense conditions. Without intervention, two specific chemical failures will occur.
Preventing High-Temperature Oxidation
The synthesis process involves melting materials at extremely high temperatures, specifically 1373 K.
At this thermal intensity, raw materials are highly reactive. If exposed to even trace amounts of air, they will oxidize rapidly.
High-vacuum sealing removes the atmosphere from the quartz tube. This isolation ensures the material remains pure and free of oxides that would otherwise degrade its thermoelectric properties.
Suppressing Volatile Component Loss
Copper sulfide materials often contain volatile components, such as sulfur and selenium.
These elements have high vapor pressures and tend to evaporate or sublime when heated. In an open or poorly sealed system, these components would escape the mixture.
The sealed quartz tube creates a containment zone. It physically prevents these volatile atoms from leaving the reaction zone, forcing them to integrate into the crystal lattice as intended.
The Impact on Material Performance
The ultimate goal of high-vacuum sealing is not just protection, but precision. The physical properties of the material are dictated by the success of this step.
Ensuring Precise Stoichiometry
"Stoichiometry" refers to the exact quantitative relationship between the constituent elements of the material.
High-performance thermoelectrics rely on a specific ratio of copper to sulfur (and selenium). If volatile components escape, this ratio shifts, altering the carrier concentration and thermal conductivity.
By preventing the loss of these elements, vacuum sealing ensures the precise stoichiometric ratio is maintained from the raw mix to the final product.
The Role of Pressure Specifics
The effectiveness of this technique relies on the quality of the vacuum.
The process specifically requires a vacuum level of 10⁻⁴ Pa.
This is not merely a "low pressure" environment; it is a high-vacuum state. Achieving this specific threshold is necessary to guarantee the complete isolation required for high-quality synthesis.
Critical Risks and Parameters
While high-vacuum sealing is the standard solution, understanding the operational boundaries is essential for success.
The Temperature-Pressure Relationship
The system must withstand the melting point of 1373 K.
The quartz tube and the seal must be robust enough to maintain the 10⁻⁴ Pa vacuum integrity even under this extreme thermal stress.
The Consequence of Seal Failure
If the vacuum seal is compromised or the pressure is insufficient (higher than 10⁻⁴ Pa), the synthesis will likely fail.
The result will be a material with uncontrolled stoichiometry (due to sulfur loss) and high impurity levels (due to oxidation), rendering it useless for high-performance applications.
Making the Right Choice for Your Synthesis
To achieve high-performance copper sulfide materials, you must treat the sealing process as a critical variable, not just a preparatory step.
- If your primary focus is Chemical Purity: Ensure your vacuum system can reliably achieve and hold 10⁻⁴ Pa to eliminate all traces of oxygen before sealing.
- If your primary focus is Compositional Accuracy: Verify the integrity of the quartz tube seal to withstand 1373 K, preventing the escape of volatile sulfur or selenium.
High-vacuum sealing is the fundamental control mechanism that transforms volatile raw ingredients into stable, high-performance thermoelectric devices.
Summary Table:
| Feature | Specification | Role in Synthesis |
|---|---|---|
| Vacuum Level | 10⁻⁴ Pa | Eliminates atmospheric oxygen and prevents oxidation |
| Max Temperature | 1373 K | Ensures structural integrity during intense melting |
| Atmosphere | Isolated Quartz | Traps volatile elements like Sulfur and Selenium |
| Result | Precise Stoichiometry | Guarantees optimal carrier concentration and performance |
Elevate Your Material Synthesis with KINTEK Expertise
Precise stoichiometry and chemical purity are the foundations of high-performance thermoelectrics. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet your unique synthesis requirements.
Whether you need to maintain a stable 10⁻⁴ Pa vacuum or reach extreme temperatures of 1373 K, our lab high-temp furnaces provide the thermal precision your research demands. Don't let oxidation or volatile loss compromise your results.
Contact KINTEK today to discuss your custom furnace needs
Visual Guide
References
- Yixin Zhang, Zhen‐Hua Ge. Synergistically optimized electron and phonon transport in high-performance copper sulfides thermoelectric materials via one-pot modulation. DOI: 10.1038/s41467-024-47148-0
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What is the primary purpose of an industrial blast drying oven for Si/HC-X? Optimize Biomass Material Pretreatment
- What are the process advantages of using template synthesis for the preparation of zinc selenide (ZnSe)?
- What is the function of controlled hot air flow treatment in ZnO drying? Master Surface Flatness and Stress Reduction
- What are the advantages of using a corundum crucible with a graphite sleeve in AlV55 alloy smelting? Ensure Pure Alloys
- How does a hybrid microwave sintering furnace compare to traditional furnaces? Optimize BZT Ceramic Production
- What is the purpose of heating a precursor solution to 80 °C and 300 rpm stirring? Achieve High-Entropy Uniformity
- Why is ALD equipment used for rear passivation of silicon solar cells? Optimize Your PERC and TOPCon Efficiency
- Why is a precision oven used to dry washed cherry pits? Unlock Superior Activated Carbon Production



















