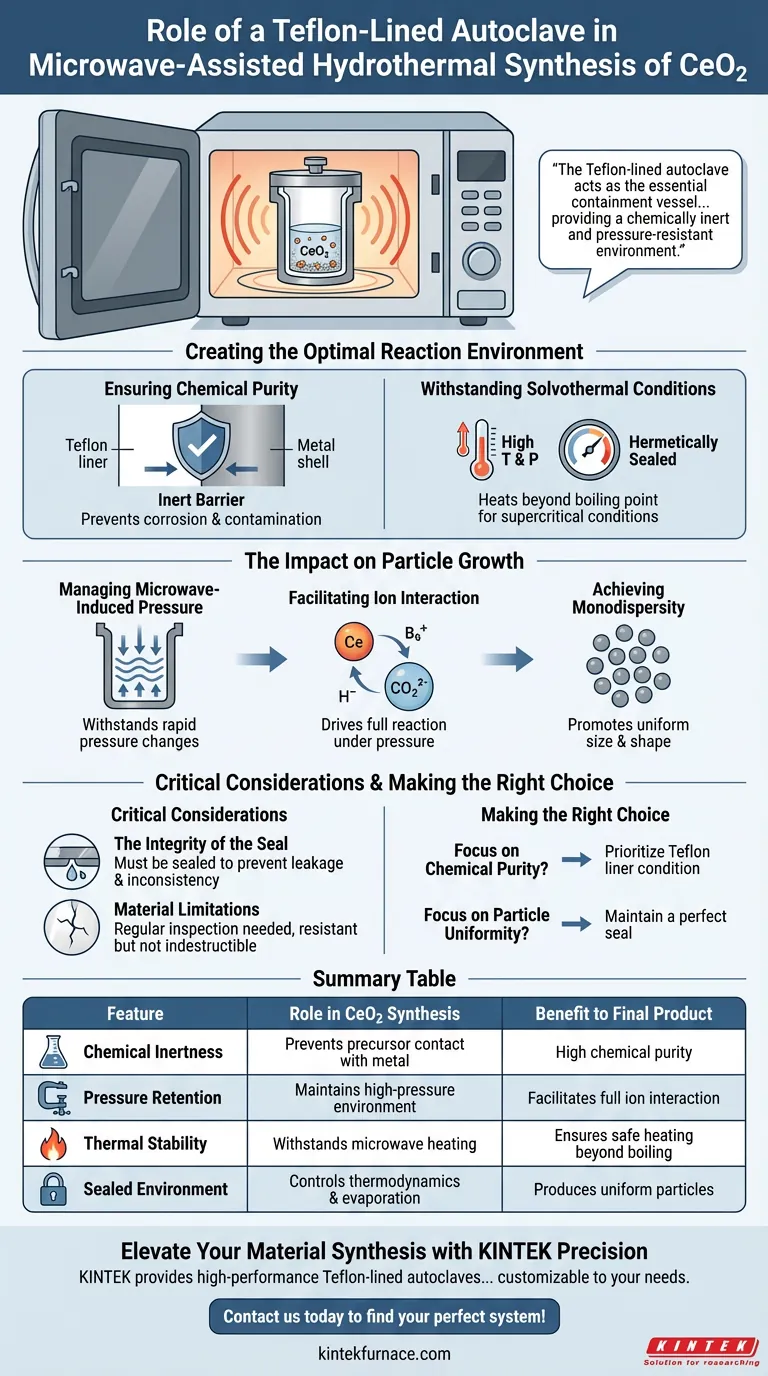
The Teflon-lined autoclave acts as the essential containment vessel for microwave-assisted hydrothermal synthesis, providing a chemically inert and pressure-resistant environment. It creates a sealed system that allows the reaction mixture to reach the high temperatures and pressures necessary to synthesize cerium dioxide (CeO2) without contamination.
The core function of the Teflon liner is to couple corrosion resistance with high-pressure retention, ensuring that the interaction between cerium and bicarbonate ions proceeds fully to yield uniform, near-monodisperse particles.

Creating the Optimal Reaction Environment
Ensuring Chemical Purity
The most immediate role of the Teflon liner is to act as a barrier between the reactive precursor solution and the metal shell of the autoclave.
Because Teflon is chemically inert, it prevents the stainless steel or alloy casing from corroding. This ensures the cerium dioxide precursor solution remains uncontaminated by metallic impurities during the synthesis process.
Withstanding Solvothermal Conditions
Microwave-assisted synthesis relies on high-temperature and high-pressure solvothermal conditions.
The autoclave provides a hermetically sealed environment. This allows the solvent to be heated well beyond its standard boiling point, creating the supercritical or near-supercritical conditions required for CeO2 formation.
The Impact on Particle Growth
Managing Microwave-Induced Pressure
Microwave heating heats the solvent directly and rapidly, which generates significant pressure changes inside the vessel.
The Teflon liner is specifically designed to withstand these internal pressure fluctuations. It maintains the structural integrity of the reaction zone, ensuring the synthesis proceeds safely and efficiently.
Facilitating Ion Interaction
The specific environment created by the autoclave is required to drive the chemical reaction to completion.
Under these high-pressure conditions, the reaction between cerium ions and bicarbonate ions is facilitated. The pressurized containment ensures that the reactants interact fully rather than evaporating or precipitating prematurely.
Achieving Monodispersity
The ultimate output of this controlled environment is the quality of the final crystal.
By maintaining a stable, high-pressure environment, the autoclave promotes the growth of near-monodisperse particles. This means the resulting CeO2 crystals are uniform in size and shape, which is a critical metric for high-quality nanomaterials.
Critical Considerations
The Integrity of the Seal
While the Teflon liner handles the chemistry, the effectiveness of the autoclave relies entirely on the seal.
If the vessel is not sealed correctly, the pressure changes generated by the microwave heating will result in leakage. This leads to inconsistent reaction conditions and a failure to achieve the desired particle uniformity.
Material Limitations
Teflon is highly resistant, but it is not indestructible.
It provides excellent corrosion resistance, but it must be inspected regularly. Physical degradation of the liner can compromise the pressure retention and introduce surface defects that could alter the nucleation of the cerium dioxide particles.
Making the Right Choice for Your Goal
The Teflon-lined autoclave is not just a container; it is an active participant in controlling the thermodynamics of your synthesis.
- If your primary focus is Chemical Purity: Prioritize the condition of the Teflon liner, ensuring it is free of defects to guarantee the inertness required to keep the precursor solution uncontaminated.
- If your primary focus is Particle Uniformity: Focus on maintaining a perfect seal to sustain the consistent high pressure needed to drive the full reaction between cerium and bicarbonate ions.
By securing the reaction environment against both contamination and pressure loss, you ensure the reproducible synthesis of high-quality cerium dioxide.
Summary Table:
| Feature | Role in CeO2 Synthesis | Benefit to Final Product |
|---|---|---|
| Chemical Inertness | Prevents precursor contact with metal casing | High chemical purity with zero metallic contamination |
| Pressure Retention | Maintains high-pressure solvothermal environment | Facilitates full interaction between cerium & bicarbonate ions |
| Thermal Stability | Withstands rapid microwave heating cycles | Ensures safe heating beyond standard solvent boiling points |
| Sealed Environment | Controls internal thermodynamics & evaporation | Produces uniform, near-monodisperse particles |
Elevate Your Material Synthesis with KINTEK Precision
Ensure the integrity of your hydrothermal processes with our advanced containment solutions. KINTEK provides high-performance Teflon-lined autoclaves, Muffle, Tube, Vacuum, and CVD systems designed to meet the rigorous demands of modern laboratories. Backed by expert R&D and manufacturing, our equipment is fully customizable to your unique research needs, ensuring maximum chemical purity and particle uniformity.
Ready to optimize your nanoparticle production? Contact us today to find your perfect system!
Visual Guide
References
- Xingzi Wang, Juanyu Yang. Controlled Synthesis of Triangular Submicron-Sized CeO2 and Its Polishing Performance. DOI: 10.3390/ma17092001
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- Are alumina ceramic furnace tubes suitable for high-pressure applications? Discover Key Factors for Safe Use
- How does an Aluminum Oxide Crucible ensure MXene purity? Key Role of LSS Etching Protection
- Why use a PLC and touch screen for magnesium vacuum distillation? For Superior Control and Safety
- What role does a laboratory vacuum pump play in a static batch desulfurization evaluation system? Ensure Data Integrity
- How does the selection of a ceramic crucible contribute to the preparation of biomass carbon catalysts? Maximize Purity
- Why is a high-precision electronic balance critical in the formulation of geopolymer binders? Precision for Success
- Why are high-purity MgO crucibles used for PbO oxidation? Essential Chemical Inertness for Master Slags
- What are the main reasons for the alumina furnace tube being prone to breaking? Prevent Costly Failures with Expert Tips



















