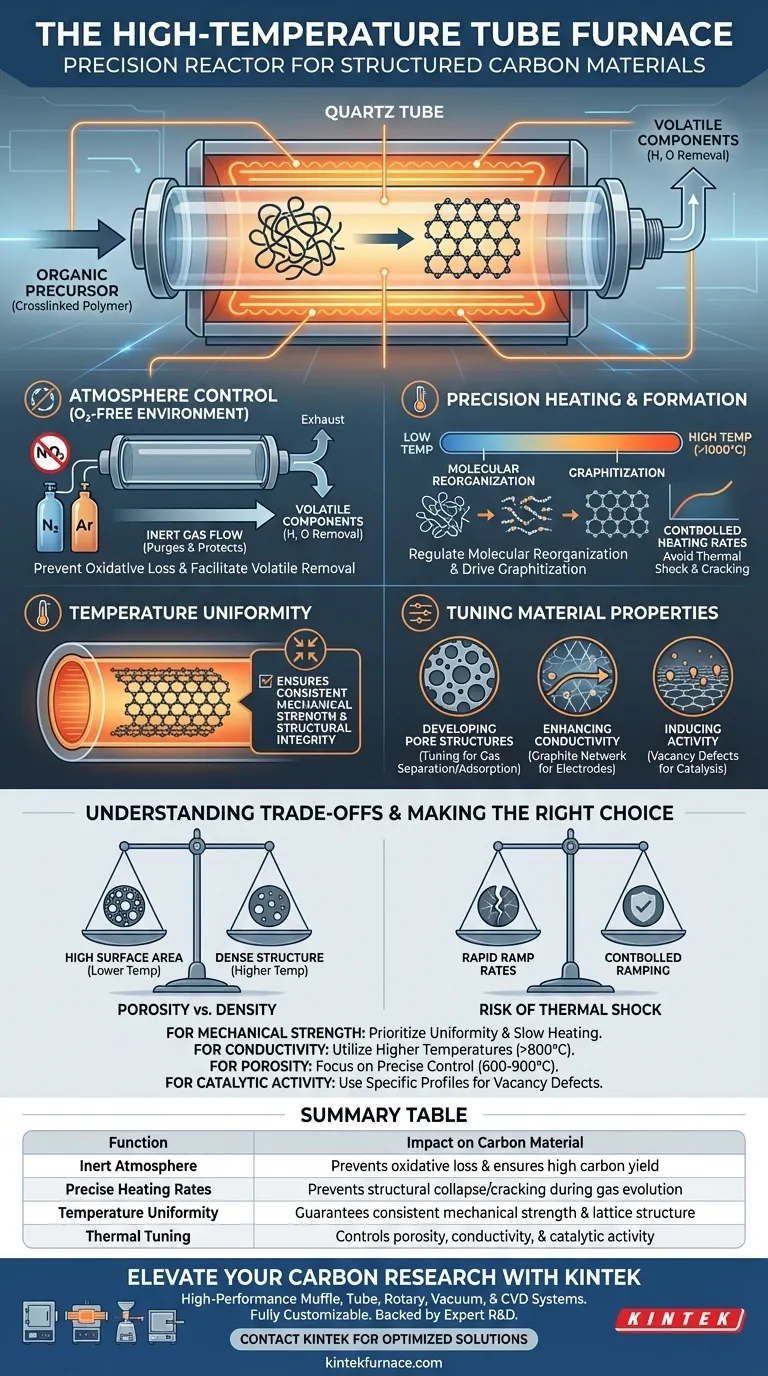
A high-temperature tube furnace acts as the precision reactor necessary to transform organic precursors into structured carbon materials without destroying them. By maintaining a strictly controlled inert environment, it allows crosslinked polymers to decompose, reorganize, and eventually graphitize into a robust carbon framework.
Core Takeaway The tube furnace is not merely a heat source; it is a tool for molecular engineering. Its ability to provide a uniform, oxygen-free atmosphere and exact heating rates is the deciding factor in the carbon yield, structural integrity, and mechanical strength of the final material.

The Critical Role of Atmosphere Control
Preventing Oxidative Loss
The most fundamental role of the furnace is to create an oxygen-free environment. If oxygen were present at high temperatures, the precursor material would simply burn to ash. By purging the tube with inert gases like high-purity nitrogen or Argon, the furnace protects the material, ensuring that mass loss is limited only to volatile components, not the carbon structure itself.
Facilitating Volatile Removal
As the furnace heats the precursor, it induces the release of non-carbon atoms (such as hydrogen and oxygen). A continuous flow of inert gas helps sweep these volatile components away from the material surface. This removal is essential for "skeletonizing" the precursor, leaving behind a pure, stable carbonized matrix.
Precision Heating and Structural Formation
Regulating Molecular Reorganization
The furnace drives the chemical transformation of the crosslinked polymer precursor. Under high heat, molecular chains break and subsequently reorganize. This process allows the remaining carbon atoms to align, eventually leading to graphitization, where the carbon forms a highly ordered, crystalline lattice.
Controlling the Rate of Change
The heating rate is a variable that must be strictly managed. Advanced tube furnaces allow for complex, multi-stage heating profiles (e.g., gradient heating). Slow, controlled ramping ensures that dehydrogenation occurs in an orderly fashion, preventing the structural collapse that can occur if gases are expelled too violently.
The Importance of Temperature Uniformity
The primary reference highlights that temperature uniformity is a critical factor for the end product. Uneven heating leads to inconsistent carbonization degrees across the sample. Uniform heat ensures that the entire batch achieves the same mechanical strength and structural integrity.
Tuning Material Properties
Developing Pore Structures
The specific temperature and heating profile determine the material's porosity. By managing the carbonization temperature (often between 600°C and 900°C), the furnace controls micropore shrinkage. This precision allows engineers to tune the pore size for specific applications, such as molecular sieving or gas separation.
Enhancing Conductivity and Activity
High-temperature treatment transforms insulating polymers into conductive carbon networks. For specific applications like electrode materials, the furnace environment can be tuned to induce carbon vacancy defects. These controlled imperfections can significantly boost the material's electrochemical activity.
Understanding the Trade-offs
The Risk of Thermal Shock
While high temperatures are necessary for graphitization, heating too quickly can be detrimental. Aggressive ramp rates may cause rapid gas evolution that cracks the material skeleton. This compromises the mechanical strength and can ruin the structural continuity required for high-performance applications.
Balancing Porosity vs. Density
There is often a trade-off between surface area and structural density. Lower temperatures may preserve more micropores (high surface area), while higher temperatures (above 1000°C) tend to collapse these pores to create a denser, more graphitic structure. The furnace settings must be chosen based on whether the priority is adsorption capacity or electrical conductivity.
Making the Right Choice for Your Goal
To optimize your carbonization process, align the furnace parameters with your specific material requirements:
- If your primary focus is Mechanical Strength: Prioritize temperature uniformity and slower heating rates to ensure a defect-free, highly ordered carbon framework.
- If your primary focus is Electrical Conductivity: Utilize higher temperatures (often >800°C) to maximize graphitization and ensure thorough removal of non-carbon atoms.
- If your primary focus is Gas Separation (Porosity): Focus on precise temperature control in the 600°C–900°C range to fine-tune micropore shrinkage and pore size distribution.
- If your primary focus is Catalytic Activity: Use specific heating profiles to induce vacancy defects within the carbon lattice, enhancing surface reactivity.
The high-temperature tube furnace is the bridge between a raw organic polymer and a functional, high-value carbon material.
Summary Table:
| Function | Impact on Carbon Material |
|---|---|
| Inert Atmosphere | Prevents oxidative loss and ensures high carbon yield |
| Precise Heating Rates | Prevents structural collapse/cracking during gas evolution |
| Temperature Uniformity | Guarantees consistent mechanical strength and lattice structure |
| Thermal Tuning | Controls porosity, conductivity, and catalytic activity |
Elevate Your Carbon Research with KINTEK
Precise carbonization requires more than just heat; it demands the absolute control found in KINTEK’s high-performance thermal systems. Backed by expert R&D and world-class manufacturing, we provide professional-grade Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous standards of material science.
Whether you are developing high-conductivity electrodes or advanced molecular sieves, our furnaces are fully customizable to your unique ramp rates and atmosphere requirements. Contact KINTEK today to discover how our engineering expertise can optimize your material’s structural integrity and performance.
Visual Guide
References
- Paul N. Smith, Zhe Qiang. Accurate additive manufacturing of lightweight and elastic carbons using plastic precursors. DOI: 10.1038/s41467-024-45211-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the role of a vacuum tube furnace during the final thermal treatment stage of Fe3O4@CSAC catalysts?
- What role does a vacuum tube furnace play as a reactor during the coal gasification reaction stage?
- What are the three main types of tube furnaces? Choose the Right One for Your Lab
- What types of heating mechanisms are employed in drop tube furnaces? Choose Between Resistive and Induction Heating
- Why is the control of heating and cooling rates in a tube furnace critical for the thermal reduction of lithium niobate?
- Why must a specialized tube annealing furnace for REBCO joint preparation use two distinct zones? Find the Perfect Balance
- What are some primary applications of the 70mm tube furnace? Unlock Precision in Materials Research
- How does a tube furnace with programmable temperature control influence gas oil catalytic cracking? Optimize Your Yield



















