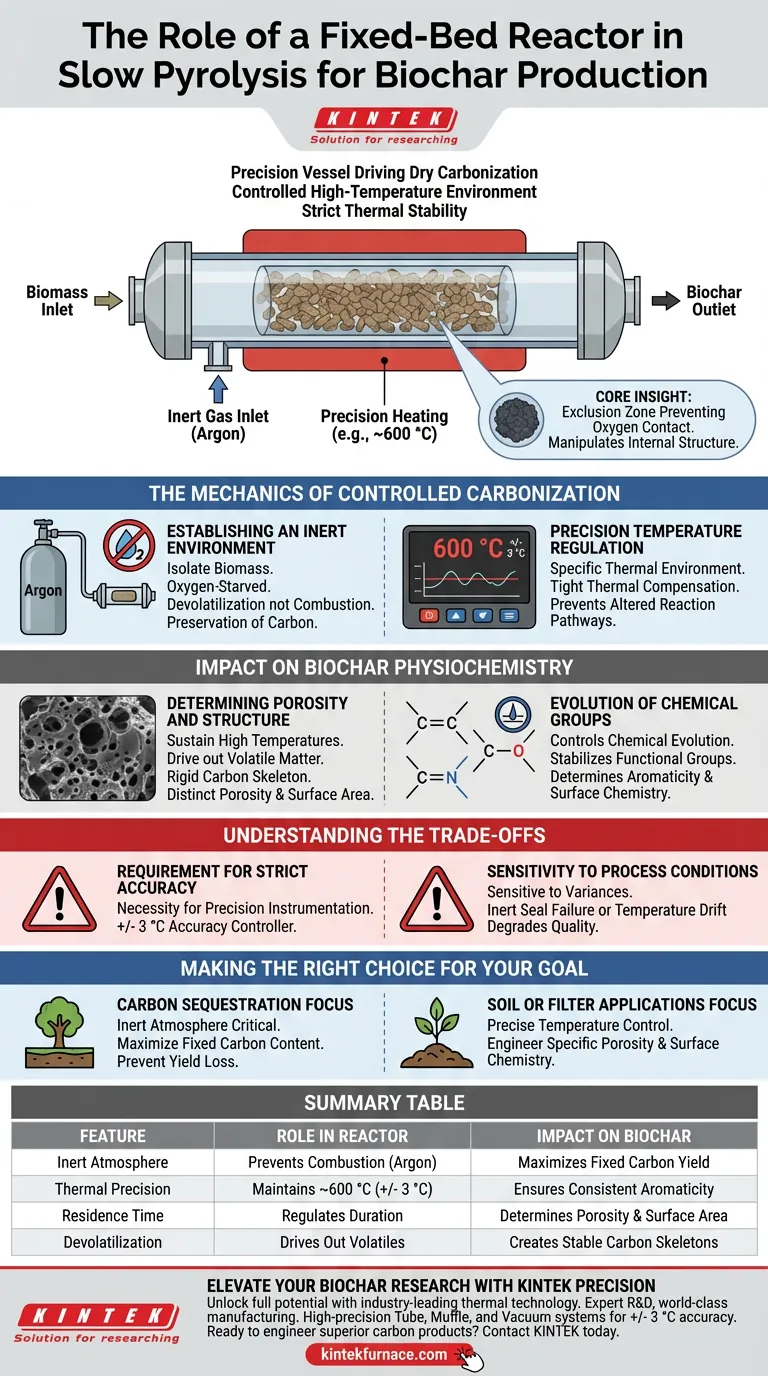
The fixed-bed reactor serves as the precision vessel that drives the dry carbonization of biomass. It creates a controlled, high-temperature environment that facilitates thermal degradation without combustion. By strictly regulating residence time and temperature, the reactor ensures the raw material is converted into solid biochar rather than ash.
Core Insight: The fixed-bed reactor is not merely a heating chamber; it is an exclusion zone that prevents oxygen contact while maintaining strict thermal stability. This precise environment is what allows for the manipulation of the biochar’s internal structure, determining its final porosity and chemical stability.

The Mechanics of Controlled Carbonization
Establishing an Inert Environment
The fundamental role of the reactor, often configured as a tube reactor, is to isolate the biomass from the outside atmosphere. By introducing an inert gas such as argon, the reactor prevents the carbon in the feedstock from reacting with oxygen.
This "oxygen-starved" state ensures that the material undergoes devolatilization rather than combustion. This preservation of carbon is essential for achieving negative carbon emission goals.
Precision Temperature Regulation
Successful slow pyrolysis relies on maintaining a specific thermal environment, often around 600 °C. The fixed-bed reactor system utilizes advanced controllers to maintain accuracy within +/- 3 °C.
This tight thermal compensation is critical. Fluctuations in temperature can alter the reaction pathway, leading to inconsistent product quality or incomplete carbonization.
Impact on Biochar Physiochemistry
Determining Porosity and Structure
The reactor’s ability to sustain high temperatures over a set residence time directly dictates the physical architecture of the biochar. The process drives out volatile matter, leaving behind a rigid carbon skeleton.
This results in a product with distinct porosity and specific surface area. These physical traits are what make biochar valuable for applications like soil amendment or filtration.
Evolution of Chemical Groups
Beyond physical structure, the reactor controls the chemical evolution of the material. The precise thermal environment stabilizes specific functional groups, such as C=C, C-O, and C-N.
The retention and transformation of these groups determine the aromaticity and surface chemistry of the final product. Without the reactor's stability, these chemical profiles would be unpredictable.
Understanding the Trade-offs
The Requirement for Strict Accuracy
The primary "cost" of using a fixed-bed reactor for high-quality biochar is the absolute necessity for precision instrumentation. The system relies on a controller capable of +/- 3 °C accuracy to ensure the stable evolution of chemical groups.
Sensitivity to Process Conditions
Because the reactor defines the product through residence time and temperature, it is highly sensitive to operational variances. A failure in the inert atmosphere seal or a drift in temperature compensation will immediately degrade the quality of the fixed carbon and alter the pore structure.
Making the Right Choice for Your Goal
The fixed-bed reactor is the tool of choice when material consistency and chemical specificity are paramount.
- If your primary focus is Carbon Sequestration: The reactor’s inert atmosphere is critical to maximize fixed carbon content and prevent yield loss through oxidation.
- If your primary focus is Soil or Filter Applications: Rely on the reactor’s precise temperature control to engineer specific porosity and surface chemistry suitable for adsorption.
The fixed-bed reactor transforms variable biomass into a predictable, engineered carbon product through rigorous environmental control.
Summary Table:
| Feature | Role in Fixed-Bed Reactor Pyrolysis | Impact on Biochar |
|---|---|---|
| Inert Atmosphere | Prevents combustion using gases like Argon | Maximizes fixed carbon yield |
| Thermal Precision | Maintains ~600 °C with +/- 3 °C accuracy | Ensures consistent chemical aromaticity |
| Residence Time | Regulates duration of thermal degradation | Determines porosity and surface area |
| Devolatilization | Drives out volatile organic compounds | Creates rigid, stable carbon skeletons |
Elevate Your Biochar Research with KINTEK Precision
Unlock the full potential of your carbonization research with KINTEK’s industry-leading thermal technology. Backed by expert R&D and world-class manufacturing, KINTEK offers high-precision Tube, Muffle, and Vacuum systems specifically engineered to maintain the strict +/- 3 °C accuracy and inert environments required for high-quality biochar production.
Whether you are developing advanced soil amendments or carbon sequestration solutions, our customizable lab high-temperature furnaces provide the stability your materials demand.
Ready to engineer superior carbon products? Contact KINTEK today to discuss your custom reactor needs.
Visual Guide
References
- Paulo André Trazzi, Witold Kwapiński. Adsorption of Ammonium, Nitrate, and Phosphate on Hydrochars and Biochars. DOI: 10.3390/app14062280
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the advantages of using an industrial-grade rapid heating furnace? Maximize Glass-Ceramic Debinding Efficiency
- Why is industrial-grade nitrogen flow introduced during the biochar pyrolysis process? Ensure Safety and Quality
- Why is precise temperature rate control in a sintering furnace vital for ceramic-sapphire composite production?
- Why is a vacuum drying oven necessary for MPCF@VG@SiNDs/C granulation? Ensure Framework Stability and Prevent Oxidation
- What functions does ammonia (NH3) perform beyond acting as a nitrogen source? Unlock Advanced Surface Engineering
- What role does a laboratory blast drying oven play in metal powder preparation? Ensure Purity & Prevent Oxidation
- How does a needle valve control silver foil surface quality for graphene growth? Prevent defects with pressure control.
- How does sodium metal function as a flux? Enhancing Sr-Ge-N Synthesis with Liquid-Phase Dynamics



















