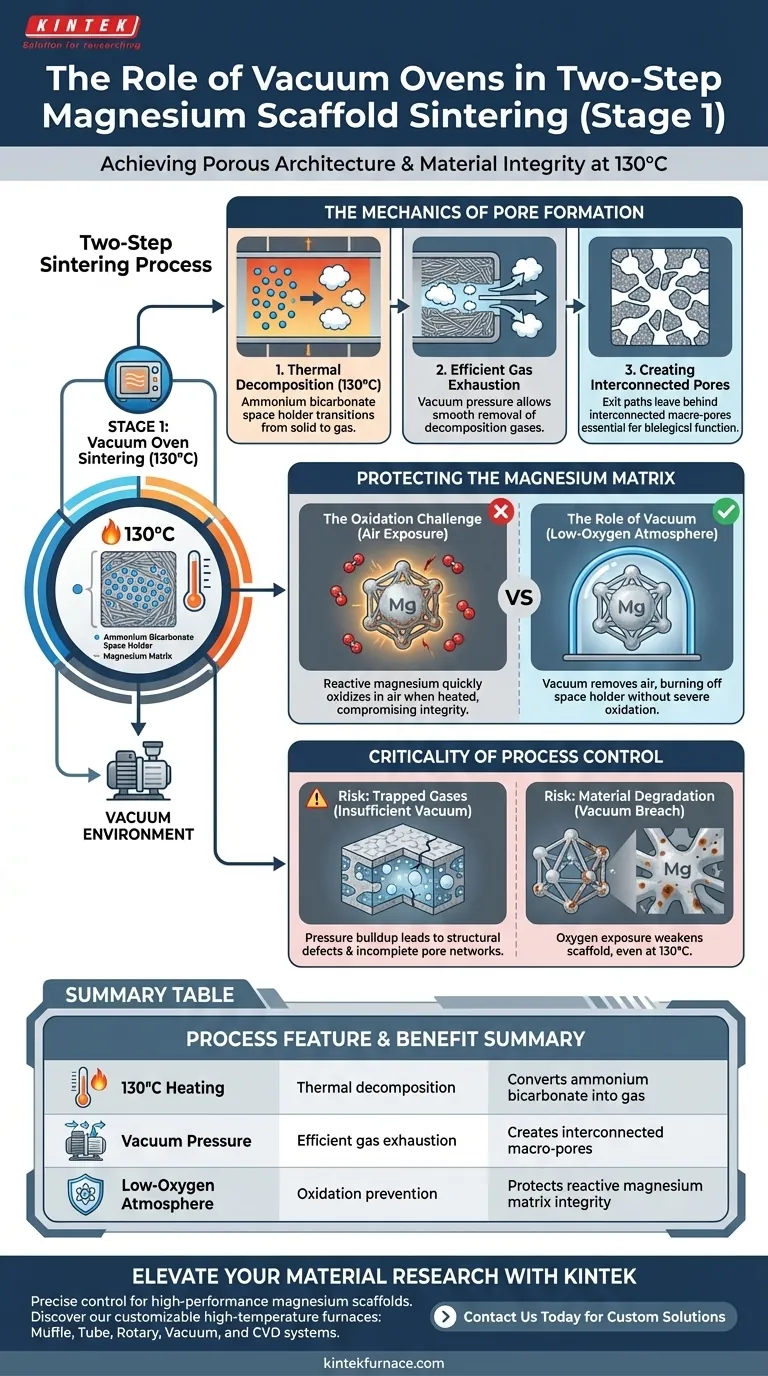
The primary purpose of using a vacuum oven in the first stage of sintering is to achieve the clean, controlled removal of the space holder material without compromising the magnesium structure. By maintaining a temperature of 130°C under vacuum, the process decomposes the ammonium bicarbonate into gas and exhausts it efficiently. This creates the necessary interconnected macro-pores while simultaneously shielding the reactive magnesium matrix from severe oxidation.
The vacuum environment performs a dual function: it facilitates the smooth evacuation of gases to establish the scaffold's porous architecture, and it creates a low-oxygen atmosphere to prevent the metallic matrix from degrading before final sintering.

The Mechanics of Pore Formation
Thermal Decomposition
The first stage focuses on the removal of the ammonium bicarbonate space holder.
The oven is set to a specific thermal environment of 130°C. At this temperature, the space holder thermally decomposes, transitioning from a solid state into gas.
Efficient Gas Exhaustion
The vacuum state is essential for managing the byproducts of decomposition.
As the space holder turns into gas, the vacuum pressure allows these gases to be exhausted smoothly from inside the compacted material.
Creating Interconnected Pores
The exit path of the gas is what defines the scaffold's structure.
As the gases escape the matrix, they leave behind voids. Because the gas is evacuated thoroughly under vacuum, these voids become interconnected macro-pores, which are critical for the biological function of the scaffold.
Protecting the Magnesium Matrix
The Oxidation Challenge
Magnesium is a highly reactive metal, especially when heated.
Without a controlled environment, heating magnesium in the presence of air would lead to rapid oxidation. This would compromise the mechanical integrity and chemical purity of the final scaffold.
The Role of Vacuum
The vacuum oven mitigates this risk by removing air from the chamber.
This creates an environment where the space holder can be burned off at 130°C without exposing the metallic matrix to severe oxidation. This preservation of the metal is vital for the success of the subsequent, higher-temperature sintering stage.
The Criticality of Process Control
Risk of Trapped Gases
If the vacuum is insufficient, decomposition gases may not evacuate smoothly.
This can lead to pressure buildup inside the scaffold, potentially causing structural defects or preventing the formation of fully interconnected pore networks.
Risk of Material Degradation
Failure to maintain the vacuum leads to immediate material degradation.
Even at the relatively low temperature of 130°C, the magnesium matrix requires protection. A breach in the vacuum seal or improper pressure levels exposes the metal to oxygen, resulting in surface contamination that weakens the scaffold.
Making the Right Choice for Your Goal
To ensure high-quality magnesium scaffolds, you must balance pore creation with material preservation.
- If your primary focus is structural connectivity: Ensure the vacuum system is capable of smooth gas exhaustion to maximize the formation of interconnected macro-pores.
- If your primary focus is material purity: Prioritize a high-integrity vacuum seal to prevent oxygen ingress and protect the metallic matrix from oxidation.
The vacuum oven is not just a heating element; it is a critical tool for sculpting the scaffold's internal architecture while preserving its chemical integrity.
Summary Table:
| Process Feature | Functional Purpose | Technical Benefit |
|---|---|---|
| 130°C Heating | Thermal decomposition | Converts ammonium bicarbonate into gas |
| Vacuum Pressure | Efficient gas exhaustion | Creates interconnected macro-pores |
| Low-Oxygen Atmosphere | Oxidation prevention | Protects reactive magnesium matrix integrity |
| Controlled Environment | Pressure management | Prevents structural defects and trapped gases |
Elevate Your Material Research with KINTEK
Precise control over vacuum and temperature is non-negotiable for high-performance magnesium scaffolds. KINTEK provides industry-leading vacuum systems designed to meet the rigorous demands of two-step sintering.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your specific laboratory requirements. Whether you are optimizing pore architecture or ensuring material purity, our high-temperature furnaces provide the stability your project needs.
Ready to refine your sintering process? Contact us today to find your custom solution.
Visual Guide
References
- Omnia Ghabour, Mona Hussein Mohy El Din. Fabrication and evaluation of the mechanical properties of reinforced biodegradable magnesium scaffolds using the space holder method. DOI: 10.21608/adjalexu.2024.290833.1507
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why is a laboratory vacuum drying oven necessary for sodium-ion battery half-cells? Achieve Peak Battery Performance
- What advantages does a vacuum drying oven offer over a standard oven for Fe3Al and CNTs? Protect Your Composites
- How does a sintering furnace optimize Al/CNT green compacts? Achieve High Densification & Precision Microstructure
- How does a high-temperature austenitization furnace ensure structural transformation? Mastering Fe-5%Mn-C Quenching
- How does the vacuum furnace body contribute to the melting process? Unlock High-Purity Metal Production
- What are the advantages of all-felt insulation? Boost Efficiency & Precision in High-Temp Processes
- What types of loads and configurations can vacuum furnaces handle? Explore Versatile Fixturing and Applications
- What is the advantage of using an integrated UHV preparation chamber? Ensure Pristine In2Se3 Surface Integrity



















