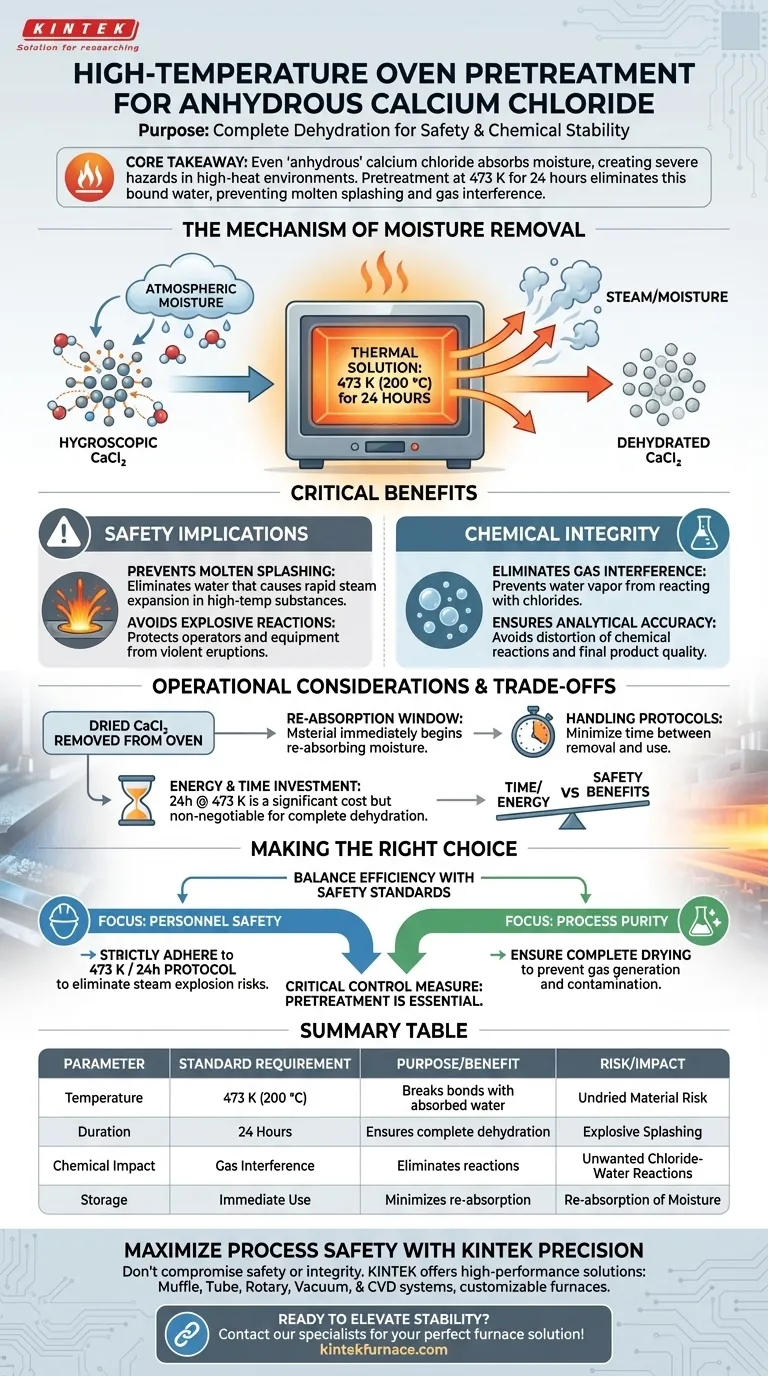
The primary purpose of using a high-temperature oven is the complete dehydration of anhydrous calcium chloride. This pretreatment process, typically conducted at 473 K for 24 hours, is essential to remove any bound water that the material has absorbed from the atmosphere. Because calcium chloride is highly hygroscopic, this step is non-negotiable for ensuring safety and chemical stability in metallurgical applications.
Core Takeaway While calcium chloride is labeled "anhydrous," it naturally pulls moisture from the air which creates severe hazards in high-heat environments. Pretreatment eliminates this water to prevent dangerous molten metal splashing and avoids the generation of interfering gases during chemical reactions.

The Mechanism of Moisture Removal
Understanding Hygroscopicity
Calcium chloride is chemically defined as hygroscopic, meaning it aggressively attracts and holds water molecules from the surrounding environment.
The Limits of "Anhydrous"
Even material purchased as "anhydrous" will accumulate bound water if exposed to air during storage or handling.
The Thermal Solution
A standard drying cycle of 24 hours at 473 K provides sufficient thermal energy to break the bonds between the calcium chloride and the absorbed water molecules, effectively driving off the moisture.
Critical Safety Implications
The Risk of Molten Splashing
The most immediate danger of introducing undried calcium chloride into a process involves its interaction with high-temperature substances, such as molten steel.
Rapid Steam Expansion
If water is present when the chemical is added to the melt, it instantly vaporizes and expands.
Preventing Explosive Reactions
This rapid expansion can cause the molten metal to splash or erupt violently, posing a severe physical threat to operators and equipment.
Preserving Chemical Integrity
Eliminating Gas Interference
Beyond physical safety, moisture introduces chemical volatility into the process.
Preventing Chloride-Water Reactions
At high temperatures, water vapor can react with chlorides to form unwanted gases.
Ensuring Analytical Accuracy
These generated gases can interfere with the intended chemical reactions or distort analytical readings, compromising the quality of the final metal product.
Operational Considerations and Trade-offs
The Re-absorption Window
Once the material is removed from the oven, it immediately begins to re-absorb moisture from the air.
Handling Protocols
Operators must minimize the time between removal from the oven and introduction to the process to maintain the benefits of pretreatment.
Energy and Time Investment
The 24-hour cycle at 473 K represents a significant time and energy cost. However, attempting to shorten this cycle to save time often results in incomplete dehydration, negating the safety benefits.
Making the Right Choice for Your Process
Ensuring the integrity of your calcium chloride is about balancing efficiency with non-negotiable safety standards.
- If your primary focus is Personnel Safety: Strictly adhere to the 473 K / 24-hour protocol to eliminate the risk of steam explosions and molten metal splashing.
- If your primary focus is Process Purity: Ensure the drying cycle is complete to prevent water vapor from reacting with chlorides and generating contaminating gases.
Pretreatment is not merely a preparatory step; it is a critical control measure for high-temperature metallurgical work.
Summary Table:
| Parameter | Standard Requirement | Purpose/Benefit |
|---|---|---|
| Temperature | 473 K (200 °C) | Breaks bonds with absorbed water molecules |
| Duration | 24 Hours | Ensures complete thermal dehydration |
| Safety Risk | Undried Material | Prevents explosive molten metal splashing |
| Chemical Impact | Gas Interference | Eliminates unwanted chloride-water reactions |
| Storage | Immediate Use | Minimizes re-absorption of atmospheric moisture |
Maximize Process Safety with KINTEK Precision
Don't compromise your lab's safety or metallurgical integrity with incomplete dehydration. KINTEK provides high-performance heating solutions designed for rigorous pretreatment protocols. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temperature furnaces—all fully customizable to meet your specific thermal energy needs.
Ready to elevate your material stability? Contact our specialists today to find your perfect furnace solution!
Visual Guide
References
- Hongyan Sun, Z. R. Chen. Copper Removal of Liquid Steel Containing 0.25% Carbon Using Fe<sub>2</sub>O<sub>3</sub>–CaCl<sub>2</sub>–SiO<sub>2</sub> Flux. DOI: 10.2355/isijinternational.isijint-2025-083
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What function does a water-cooling system serve in muffle furnaces? Stability & Precision Secrets Revealed
- What is the primary function of a muffle furnace in iron-modified activated carbon prep? Optimize Adsorption Sites
- What is the function of a muffle furnace during catalyst calcination? Master Biomass-to-Catalyst Transformation
- What should be avoided when operating a muffle furnace? Key Safety Tips to Prevent Damage and Hazards
- What is the specific application of a muffle furnace in biochar characterization experiments? Optimize Ash Analysis
- Why is annealing in a 600 Celsius muffle furnace critical for ZnCo2O4? Unlock High-Performance Spinel Catalysts
- How does the price of a muffle furnace vary? Find the Perfect Fit for Your Lab's Budget
- What role does a high-precision high-temperature box furnace play in the controlled foaming of aluminum? Key Insights



















