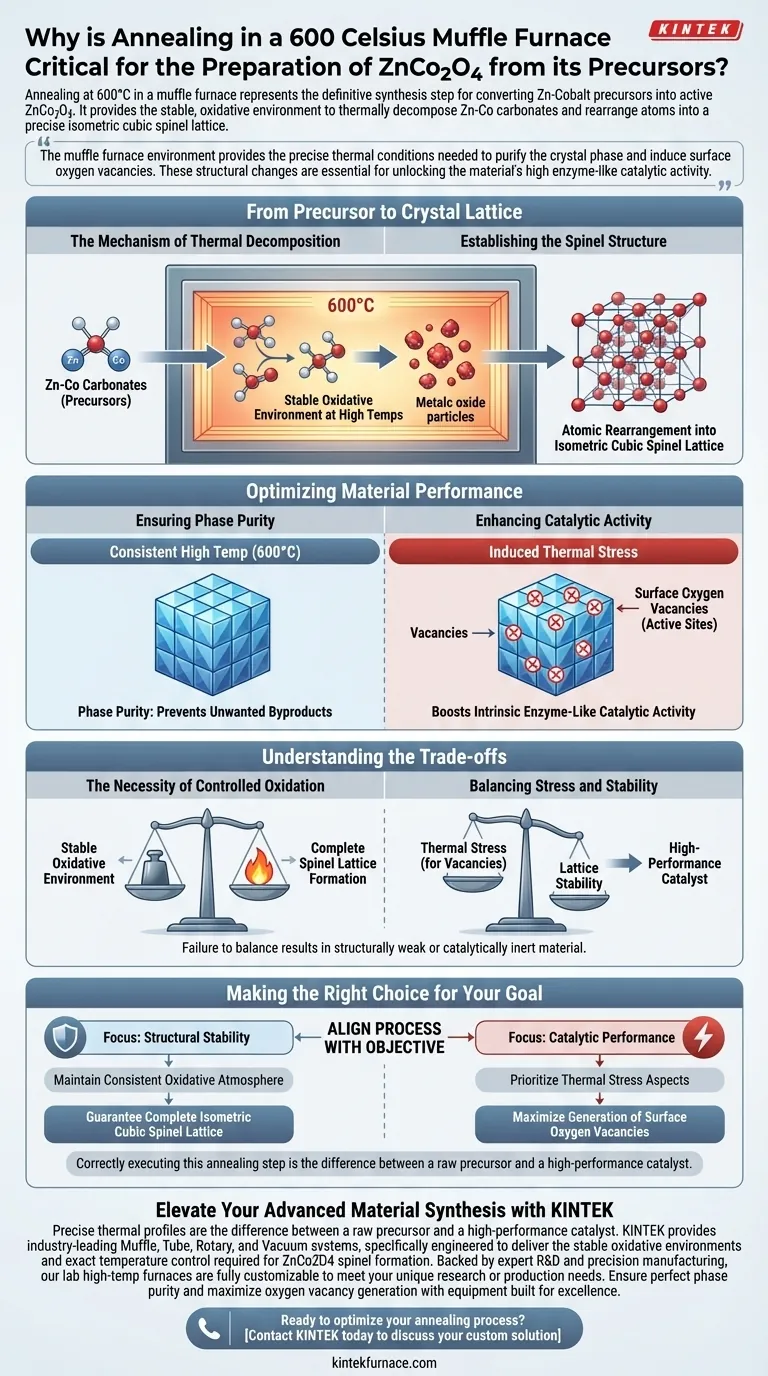
Annealing in a muffle furnace at 600°C represents the definitive synthesis step for converting zinc-cobalt precursors into active ZnCo2O4. It provides the stable, oxidative environment necessary to thermally decompose Zn-Co carbonates and rearrange the atomic structure into a precise isometric cubic spinel lattice.
The muffle furnace environment provides the precise thermal conditions needed to purify the crystal phase and induce surface oxygen vacancies. These structural changes are essential for unlocking the material's high enzyme-like catalytic activity.

From Precursor to Crystal Lattice
The Mechanism of Thermal Decomposition
The primary function of the muffle furnace is to facilitate the thermal decomposition of Zn-Co carbonate precursors.
At high temperatures, the furnace creates a stable oxidative environment. This allows the carbonate components to break down consistently, leaving behind the desired metallic oxides.
Establishing the Spinel Structure
Once the precursors decompose, the remaining atoms must be organized correctly.
The annealing process drives a critical rearrangement of atoms. This transforms the raw material into a stable isometric cubic spinel lattice, which constitutes the structural backbone of ZnCo2O4.
Optimizing Material Performance
Ensuring Phase Purity
The muffle furnace treatment is the deciding factor in the material's final composition.
By maintaining a consistent high temperature (600°C), the process ensures phase purity. This prevents the formation of unwanted byproducts that could interfere with the material's function.
Enhancing Catalytic Activity
The most critical outcome of this annealing process is the enhancement of chemical reactivity.
The thermal stress induced during annealing generates abundant oxygen vacancies on the oxide surface. These vacancies act as active sites, significantly boosting the intrinsic enzyme-like catalytic activity of the final product.
Understanding the Trade-offs
The Necessity of Controlled Oxidation
The criticality of the muffle furnace lies in its ability to provide a stable oxidative environment.
If the heating environment is inconsistent or lacks sufficient oxygen, the rearrangement into the spinel lattice may remain incomplete. This would result in a material with poor structural integrity and low catalytic potential.
Balancing Stress and Stability
While thermal stress is necessary to create oxygen vacancies, it must be carefully controlled.
The process relies on a specific thermal profile to generate these vacancies without destroying the lattice. Failing to achieve this balance results in a material that is either structurally weak or catalytically inert.
Making the Right Choice for Your Goal
To maximize the quality of your ZnCo2O4 preparation, align your process with your specific objectives:
- If your primary focus is Structural Stability: Ensure the furnace maintains a consistent oxidative atmosphere to guarantee the complete formation of the isometric cubic spinel lattice.
- If your primary focus is Catalytic Performance: Prioritize the thermal stress aspects of the annealing phase to maximize the generation of surface oxygen vacancies.
Correctly executing this annealing step is the difference between a raw precursor and a high-performance catalyst.
Summary Table:
| Process Objective | Mechanism | Outcome for ZnCo2O4 |
|---|---|---|
| Precursor Conversion | Thermal Decomposition | Breaks down Zn-Co carbonates into metallic oxides |
| Structural Formation | Atomic Rearrangement | Establishes a stable isometric cubic spinel lattice |
| Purity Control | High-Temp Oxidation | Eliminates unwanted byproducts and ensures phase purity |
| Performance Boost | Induced Thermal Stress | Generates surface oxygen vacancies for catalytic activity |
Elevate Your Advanced Material Synthesis with KINTEK
Precise thermal profiles are the difference between a raw precursor and a high-performance catalyst. KINTEK provides industry-leading Muffle, Tube, Rotary, and Vacuum systems, specifically engineered to deliver the stable oxidative environments and exact temperature control required for ZnCo2O4 spinel formation.
Backed by expert R&D and precision manufacturing, our lab high-temp furnaces are fully customizable to meet your unique research or production needs. Ensure perfect phase purity and maximize oxygen vacancy generation with equipment built for excellence.
Ready to optimize your annealing process? Contact KINTEK today to discuss your custom solution.
Visual Guide
References
- Shu-Ju Liao, Zhong Cao. Pt@ZnCo2O4 Microspheres as Peroxidase Mimics: Enhanced Catalytic Activity and Application for L-Cysteine Detection. DOI: 10.3390/molecules30010187
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What advantages do rapid heating and cooling features offer in some muffle furnace models? Boost Efficiency and Control in Your Lab
- What are the safety procedures for loading and unloading samples in a muffle furnace? Ensure Operator and Equipment Safety
- What are the key advantages of using muffle furnaces? Achieve Clean, Uniform, and High-Temp Heating
- What is the significance of muffle furnaces in research and analysis? Unlock Precise Heat Treatment for Your Lab
- What role does a high-temperature muffle furnace play in the preparation of acid-activated clay? Key Thermal Mechanisms
- What is the function of a high-temperature muffle furnace in the columbite precursor method? Pure Perovskite Synthesis
- What is the role of an industrial-grade high-temperature box furnace in Ni-Ti-Hf-La alloy post-processing?
- What makes box furnaces versatile equipment? Unlock Adaptable Heating for Diverse Applications



















