The primary purpose of long-duration calcination in a high-temperature box resistance furnace is to drive critical solid-state reactions that transform raw precursors into a stable, active catalyst. This process utilizes a stable high-temperature environment (typically around 1050 °C) to simultaneously purify the material by removing organic networks and engineer its internal structure through atomic diffusion.
Core Takeaway: High-temperature calcination is not merely a drying step; it is a structural engineering process. It provides the necessary thermal energy to burn off organic templates and force metal ions to diffuse into specific lattice sites, creating a well-crystallized and chemically active perovskite phase.

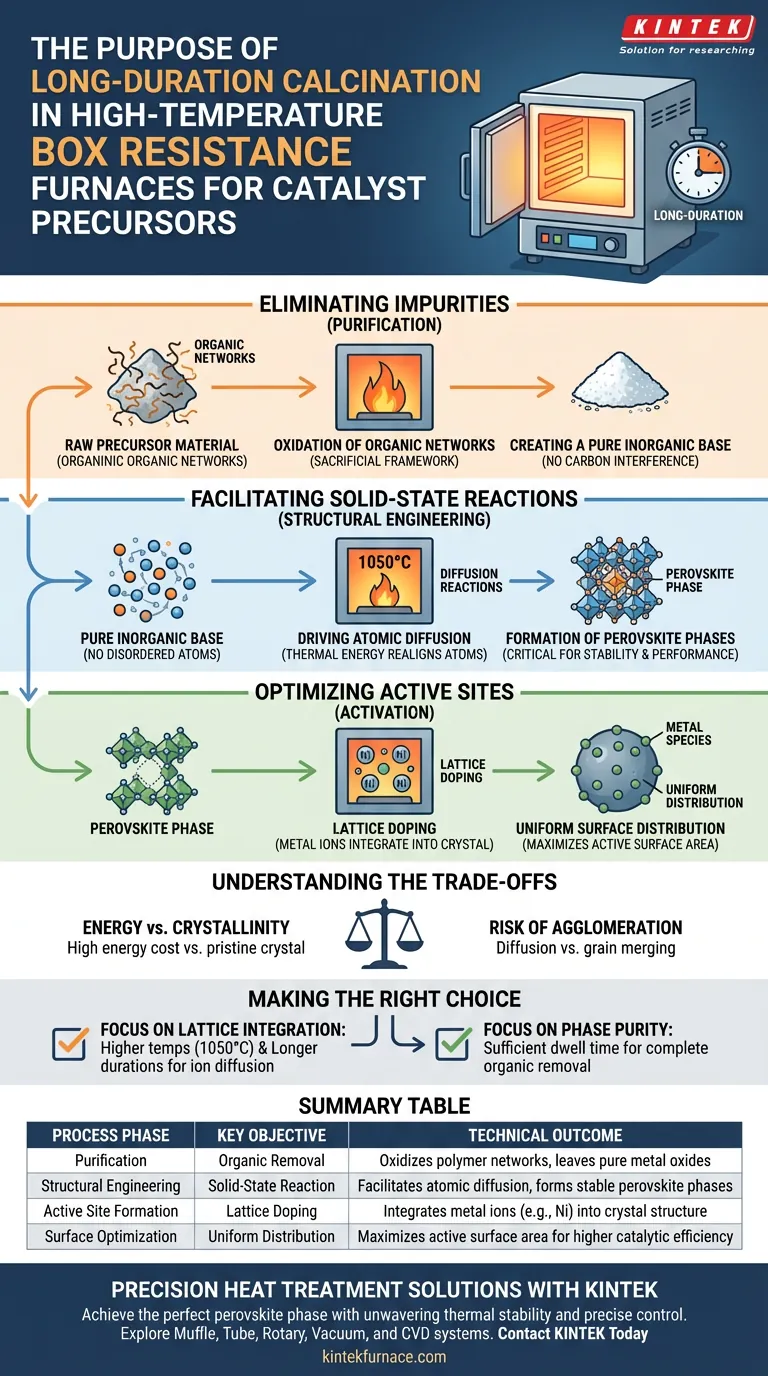
Eliminating Impurities and Templates
The first function of the furnace is to act as a purification chamber. Before the final crystal structure can form, the material must be stripped of the organic components used during the initial synthesis.
Oxidation of Organic Networks
During the thermal treatment, the organic polymer network acts as a sacrificial framework. The high heat facilitates the complete oxidation of these polymers, effectively removing them from the matrix.
Creating a Pure Inorganic Base
By burning off these organic residues, the furnace leaves behind only the essential metal oxides. This ensures that the final catalyst consists solely of the desired inorganic material without carbonaceous interference.
Facilitating Solid-State Reactions
Once the impurities are removed, the sustained high temperature drives the physical transformation of the material. This is where the box resistance furnace’s ability to maintain stable heat becomes critical.
Driving Atomic Diffusion
At temperatures such as 1050 °C, the material undergoes diffusion reactions. The thermal energy allows atoms to move within the solid state, rearranging themselves from a disordered mixture into a highly ordered structure.
Formation of Perovskite Phases
The ultimate goal of this diffusion is the crystallization of specific material phases. The long-duration treatment ensures the formation of a well-crystallized perovskite phase, which is often required for the catalyst's stability and performance.
Optimizing Active Sites
Beyond forming the base structure, calcination is responsible for activating the catalyst. This involves the precise placement of active metal species within or upon the material.
Lattice Doping
The process effectively dopes nickel species (or other active metals) directly into the crystal lattice. The high temperature forces these ions into specific positions within the structure, enhancing the catalyst's intrinsic activity.
Uniform Surface Distribution
In addition to lattice integration, the thermal treatment promotes the uniform distribution of metal species on the particle surfaces. This maximizes the surface area available for catalytic reactions, ensuring high efficiency.
Understanding the Trade-offs
While long-duration high-temperature calcination is essential for crystallinity and doping, it presents specific challenges that must be managed.
Energy vs. Crystallinity
Achieving a well-crystallized perovskite phase at 1050 °C is highly energy-intensive. One must balance the cost of long-duration heating against the requirement for a pristine crystal structure.
Risk of Agglomeration
While high temperatures drive diffusion, they can also cause grains to merge. Precise temperature control is required to achieve the desired phase without causing excessive agglomeration, which would reduce the active surface area.
Making the Right Choice for Your Goal
To maximize the effectiveness of your synthesis, align your calcination parameters with your specific material requirements.
- If your primary focus is Lattice Integration: Prioritize higher temperatures (e.g., 1050 °C) and longer durations to ensure sufficient energy for metal ions like Nickel to diffuse into the crystal structure.
- If your primary focus is Phase Purity: Ensure the dwell time is sufficient to fully oxidize and remove the organic polymer network before cooling.
Successful catalyst synthesis relies on viewing the furnace not just as a heater, but as a precision tool for atomic-level construction.
Summary Table:
| Process Phase | Key Objective | Technical Outcome |
|---|---|---|
| Purification | Organic Removal | Oxidizes polymer networks to leave pure metal oxides |
| Structural Engineering | Solid-State Reaction | Facilitates atomic diffusion to form stable perovskite phases |
| Active Site Formation | Lattice Doping | Integrates metal ions (e.g., Ni) into the crystal structure |
| Surface Optimization | Uniform Distribution | Maximizes active surface area for higher catalytic efficiency |
Precision Heat Treatment Solutions with KINTEK
Achieving the perfect perovskite phase requires unwavering thermal stability and precise control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of catalyst synthesis. Whether you need to drive complex solid-state reactions or ensure uniform lattice doping, our lab high-temperature furnaces are fully customizable for your unique research needs.
Ready to enhance your lab's synthesis efficiency? Contact KINTEK Today to explore our specialized furnace solutions.
Visual Guide
References
- Lan Zhang, Saifudin Abubakar. Catalytic decomposition of methane: Ni-promoted perovskite oxide catalysts for turquoise hydrogen and carbon nanomaterials Co-production. DOI: 10.20517/energymater.2024.53
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What process conditions must a muffle furnace satisfy for CoNiCrAlY oxidation? Ensure Precise High-Temp Stability
- What are the temperature capabilities of muffle furnaces? Find Your Perfect High-Temp Solution
- Why is a laboratory high-temperature muffle furnace required for 900°C (Nd,Gd)1/3Sr2/3CoO3-d cathode treatment?
- Why are muffle furnaces particularly useful in material science? Unlock Precise, Contaminant-Free Heat Treatment
- What safety measures should be taken when handling thermocouples in a muffle furnace? Essential Tips for Safe Operation
- What role does an electric muffle furnace play in the siliconization of 10Kh23N18 steel welds? Expert Thermal Insight
- What is a Muffle furnace and what are its main characteristics? Discover High-Purity Heating Solutions
- How does a forced convection oven facilitate the curing of flame-retardant epoxy resin? Ensure Uniform Cross-Linking



















