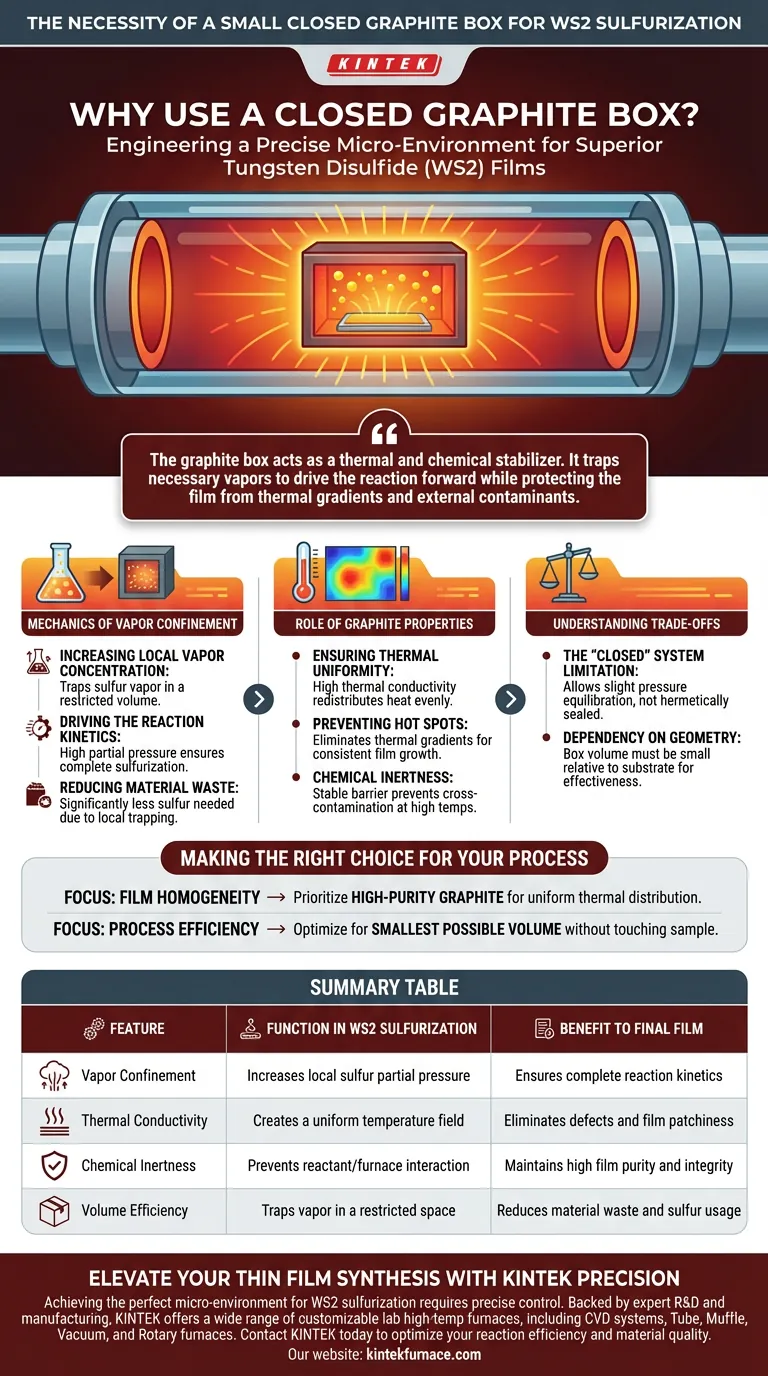
The necessity of a small closed graphite box lies in its ability to engineer a precise micro-environment. It functions as a containment vessel that drastically increases the local concentration of sulfur vapor while ensuring the reaction occurs under a uniform temperature field. Without this confinement, sulfur vapor would dissipate rapidly, leading to inconsistent film formation and excessive material waste.
The graphite box acts as a thermal and chemical stabilizer. It traps necessary vapors to drive the reaction forward while protecting the film from thermal gradients and external contaminants.

The Mechanics of Vapor Confinement
Increasing Local Vapor Concentration
The primary function of the graphite box is to create a restricted volume around the reactants. By confining the space, the box traps sulfur vapor that sublimates at high temperatures.
Driving the Reaction Kinetics
This containment significantly increases the local partial pressure of sulfur surrounding the substrate. A high concentration of vapor is thermodynamically necessary to ensure the complete sulfurization of the tungsten precursor into Tungsten Disulfide (WS2).
Reducing Material Waste
Because the vapor is trapped locally rather than dispersed into the larger furnace tube, the process becomes much more efficient. You require a significantly smaller amount of sulfur powder to achieve the necessary saturation levels.
The Role of Graphite Properties
Ensuring Thermal Uniformity
Graphite possesses high thermal conductivity, which is critical for thin film growth. The box absorbs heat from the furnace and redistributes it evenly across the substrate.
Preventing Hot Spots
This redistribution creates a uniform temperature field within the box. Eliminating thermal gradients ensures that the WS2 film grows consistently across the entire surface area, avoiding patchy or structurally weak regions.
Chemical Inertness
Graphite is chemically stable at the high temperatures required for sulfurization. It acts as a neutral barrier that prevents cross-contamination between the reactants and the furnace environment.
Understanding the Trade-offs
The "Closed" System Limitation
While the box is described as "closed," it is generally not hermetically sealed; it must allow for slight pressure equilibration while retaining the bulk of the vapor. If the box is sealed too tightly, pressure buildup could alter the reaction kinetics unpredictably.
Dependency on Geometry
The effectiveness of this method relies heavily on the "small" nature of the box relative to the substrate. If the box volume is too large, the vapor pressure will drop, negating the benefits of confinement and potentially leading to incomplete sulfurization.
Making the Right Choice for Your Process
To maximize the quality of your WS2 films, consider the following regarding your experimental setup:
- If your primary focus is film homogeneity: Prioritize the quality of the graphite; high-purity graphite ensures the most uniform thermal distribution.
- If your primary focus is process efficiency: Optimize the box volume to be as small as possible without touching the sample to minimize sulfur powder usage.
By controlling the vapor pressure and temperature profile simultaneously, the graphite box transforms an unpredictable open system into a reliable synthesis reactor.
Summary Table:
| Feature | Function in WS2 Sulfurization | Benefit to Final Film |
|---|---|---|
| Vapor Confinement | Increases local sulfur partial pressure | Ensures complete reaction kinetics |
| Thermal Conductivity | Creates a uniform temperature field | Eliminates defects and film patchiness |
| Chemical Inertness | Prevents reactant/furnace interaction | Maintains high film purity and integrity |
| Volume Efficiency | Traps vapor in a restricted space | Reduces material waste and sulfur usage |
Elevate Your Thin Film Synthesis with KINTEK Precision
Achieving the perfect micro-environment for WS2 sulfurization requires more than just high temperatures—it requires precise control. Backed by expert R&D and manufacturing, KINTEK offers a wide range of lab high-temp furnaces, including CVD systems, Tube, Muffle, Vacuum, and Rotary furnaces, all of which can be customized for your unique graphite box configurations and material needs.
Whether you are a researcher or an industrial manufacturer, our systems provide the thermal stability and atmospheric control necessary for superior thin film growth. Contact KINTEK today to discuss how our customizable high-temperature solutions can optimize your reaction efficiency and material quality.
Visual Guide
References
- F. Sava, Alin Velea. Synthesis of WS2 Ultrathin Films by Magnetron Sputtering Followed by Sulfurization in a Confined Space. DOI: 10.3390/surfaces7010008
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why are high-purity crucibles and sealed reaction environments necessary for NaRu2O4 growth? Ensure Crystal Integrity
- Why is a high-purity Alumina Crucible required during the annealing of MoS2? Ensure High-Temperature Material Purity
- What is the purpose of a water-cooled condenser in a thermal vacuum mercury removal apparatus? Key for Safe Recovery
- What mechanical properties should be evaluated for alumina ceramic furnace tubes? Ensure Durability and Performance
- How do cooling modules in high-temperature laboratory furnaces manage thermal energy? Protect Your System Components
- What are the advantages of 0.7 mm quartz capillaries for SXRD? Optimize High-Energy In-Situ X-ray Experiments
- Why is a molybdenum crucible considered an ideal choice for quartz melting? High-Purity Solutions at 2000°C
- Why use high-alumina (Alundum) crucibles for monazite glass-ceramic synthesis? Ensure Purity in High-Heat Research



















