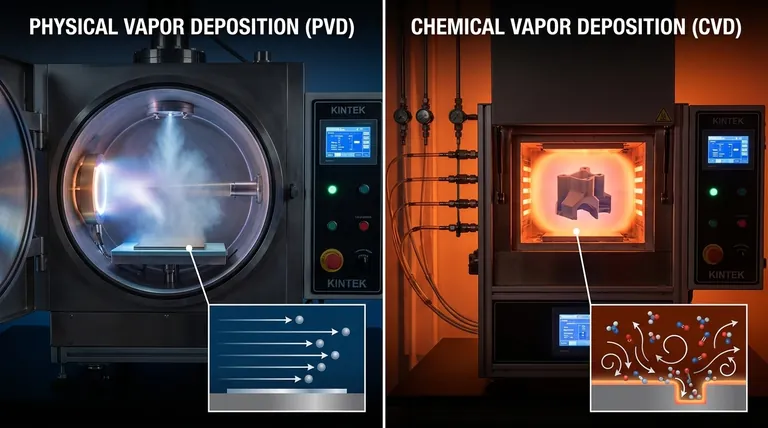
At its core, the difference between Physical Vapor Deposition (PVD) and Chemical Vapor Deposition (CVD) lies in how the coating material arrives on the substrate. PVD is a physical process where a solid material is vaporized and then condenses onto the part, much like steam condensing on a cold mirror. CVD is a chemical process where reactive gases are introduced into a chamber, and they react with each other on the substrate's surface to form a new, solid coating layer.
The choice between PVD and CVD is a fundamental engineering decision driven by a key trade-off. PVD offers superior film purity and operates at lower temperatures, but it is a "line-of-sight" process. CVD provides exceptional, uniform coverage on complex shapes but typically requires high temperatures and involves chemical reactions that can introduce impurities.
The Fundamental Process: Physical vs. Chemical
To select the right method, you must first understand the distinct mechanisms at play. The names themselves—Physical Vapor Deposition versus Chemical Vapor Deposition—are the most accurate descriptors of what is happening at the atomic level.
How PVD Works: A Physical Transfer
PVD is a mechanical or thermal process. It begins with a solid source material, often called a "target," inside a high-vacuum chamber.
Energy is applied to this target, causing it to vaporize into atoms or molecules. This is typically done through sputtering (bombarding the target with ions) or thermal evaporation (heating it until it vaporizes).
These vaporized particles travel in a straight line through the vacuum and condense directly onto the cooler substrate, forming a thin, solid film. The coating is the exact same material as the source target.
How CVD Works: A Chemical Creation
CVD begins not with a solid, but with one or more volatile precursor gases that contain the elements you wish to deposit.
These gases are fed into a reaction chamber containing the substrate. Energy, usually in the form of high heat, is applied to the system.
This energy triggers a chemical reaction on or near the substrate's surface. The reaction causes the gases to decompose and form a new, solid material that grows directly on the substrate, with other chemical byproducts being exhausted from the chamber.
Key Differentiators in Practice
The difference between a physical transfer and a chemical reaction creates significant practical consequences for temperature, coverage, and final film quality.
Operating Temperature and Substrate Impact
CVD generally requires very high temperatures, often ranging from several hundred to over 1000°C, to provide the activation energy needed for the chemical reactions to occur. This severely limits the types of substrates that can be coated, excluding most plastics and certain metals.
PVD, by contrast, can be performed at much lower temperatures, typically from room temperature to a few hundred degrees Celsius. This makes it far more versatile for coating heat-sensitive materials.
A key exception is Plasma-Enhanced CVD (PECVD), a variant that uses plasma to excite the gases. This allows the chemical reactions to proceed at much lower temperatures, bridging the gap between traditional CVD and PVD.
Conformality and Coverage
Conformality refers to a coating's ability to uniformly cover complex shapes, including sharp edges, trenches, and internal surfaces. This is where CVD holds a decisive advantage.
Because CVD relies on precursor gases that can flow and diffuse freely, it can coat intricate 3D geometries and even the inside of a hollow part with exceptional uniformity.
PVD is a line-of-sight process. The vaporized material travels in a straight path from the source to the substrate. Any surface not directly in this line of sight will receive little to no coating, creating a "shadowing" effect. This makes PVD poorly suited for parts with complex geometries.
Purity and Film Quality
PVD processes, conducted in a high-vacuum environment from a solid, often pure source, tend to produce films with very high purity and density. You have direct control over the deposited material's composition.
CVD films can sometimes incorporate impurities from the precursor gases or from unreacted byproducts of the chemical reaction. The film structure may also be less dense than a comparable PVD film.
Understanding the Trade-offs
Neither method is universally superior. The optimal choice is always a compromise based on the specific application's requirements.
The PVD Compromise: Line-of-Sight vs. Purity
With PVD, you gain exceptional purity, density, and a wide selection of compatible substrates due to lower process temperatures. The price for this is poor conformality, limiting its use primarily to flat or gently curved surfaces that can be directly faced toward the source.
The CVD Compromise: Conformality vs. Conditions
With CVD, you gain outstanding, uniform coverage on even the most complex parts. The price for this is the need for very high temperatures that can damage or warp the substrate and the risk of incorporating chemical impurities into the final film.
Making the Right Choice for Your Application
The best method depends entirely on your project's non-negotiable requirements. Use these guidelines to make a clear decision.
- If your primary focus is coating a complex 3D part or internal surface: CVD is almost always the superior choice due to its excellent conformality.
- If your primary focus is achieving the highest film purity and density on a relatively flat surface: PVD is the ideal method, offering precise control in a clean vacuum environment.
- If your primary focus is coating a heat-sensitive material like a polymer: PVD is the safer default, though specialized low-temperature CVD processes (like PECVD) should also be considered.
By understanding the core distinction between physical transfer and chemical reaction, you can confidently select the deposition technique that aligns perfectly with your engineering goals.
Summary Table:
| Aspect | PVD | CVD |
|---|---|---|
| Process Type | Physical vaporization and condensation | Chemical reaction of gases on substrate |
| Temperature | Low (room temp to a few hundred °C) | High (several hundred to over 1000°C) |
| Coverage | Line-of-sight, poor for complex shapes | Excellent conformality on 3D geometries |
| Purity | High purity and density | Potential for impurities from reactions |
| Substrate Compatibility | Versatile, good for heat-sensitive materials | Limited to high-temperature resistant substrates |
Struggling to choose between PVD and CVD for your lab's coating needs? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including CVD/PECVD Systems and more. Our deep customization capabilities ensure we meet your unique experimental requirements with precision. Contact us today to discuss how our tailored solutions can enhance your deposition processes and achieve superior results!
Visual Guide
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- MPCVD Machine System Reactor Bell-jar Resonator for Lab and Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano Diamond Coating
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- Why is the temperature control system important in MPCVD equipment? Ensure Precise Diamond Growth and Process Stability
- Why is keeping maintenance records important for MPCVD equipment? Ensure Reliability and Quality in Crystal Growth
- Why is maintaining gas pipelines important in MPCVD equipment? Ensure Purity and Safety in Crystal Growth
- How is MPCVD used in the production of polycrystalline diamond optical components? Discover High-Purity Diamond Growth for Optics
- Who should perform maintenance on MPCVD equipment? Trust Certified Experts for Safety and Precision



















