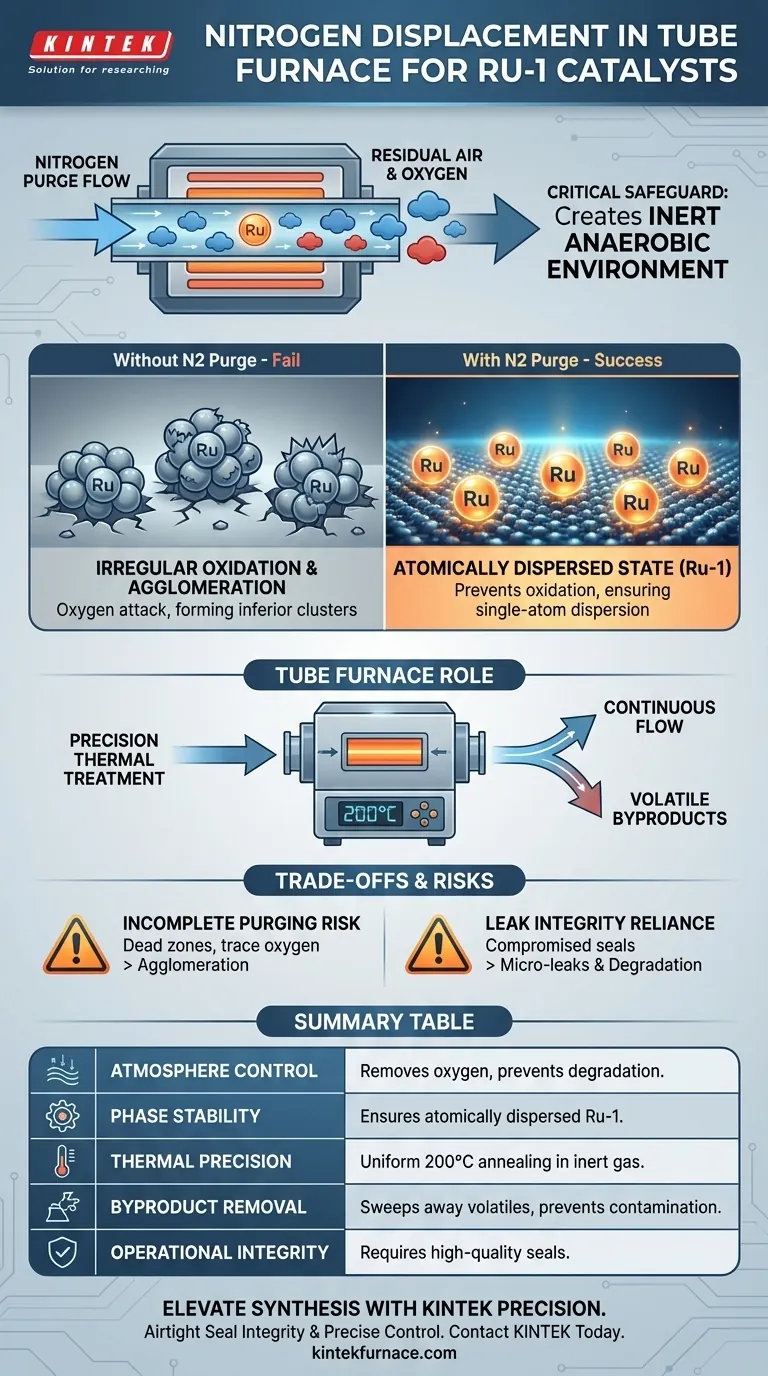
The nitrogen displacement step serves as the critical safeguard for catalyst structural integrity. It functions to systematically purge residual air from the Tube Furnace chamber, replacing it with a continuous flow of inert gas. This ensures that the subsequent thermal treatment occurs in a strictly anaerobic environment, which is a prerequisite for synthesizing high-quality ruthenium-1 (Ru-1) catalysts.
Core Takeaway The presence of oxygen during the heating of ruthenium complexes leads to irreversible material degradation. Nitrogen displacement prevents irregular oxidation and metal agglomeration, ensuring the precursors resolve into an atomically dispersed state (Ru-1) rather than forming catalytically inferior clusters.

The Physics of Atmosphere Control
Creating an Anaerobic Environment
The primary mechanical function of the nitrogen displacement step is the complete removal of oxygen from the furnace chamber.
Before any heating begins, a continuous flow of inert nitrogen gas flushes out ambient air. This establishes a baseline environment where chemical reactions are driven solely by thermal energy, not by unwanted oxidative interactions with the atmosphere.
Preventing Irregular Oxidation
Ruthenium complexes are highly sensitive to oxygen, particularly as temperatures rise.
Without the nitrogen purge, residual oxygen attacks the precursor materials. This leads to irregular oxidation, where the chemical structure of the catalyst is altered unpredictably, destroying the intended catalytic properties before they are even formed.
Ensuring Atomic Dispersion
The ultimate goal of this specific preparation method is to create an atomically dispersed state, known as Ru-1.
If oxygen is present, the metal atoms tend to migrate and clump together. By maintaining an inert atmosphere, the nitrogen step "freezes" the dispersion, ensuring the ruthenium remains as isolated single atoms rather than aggregating into larger, less active nanoparticles.
The Role of the Tube Furnace
Precision Thermal Treatment
The Tube Furnace is essential because it couples this atmosphere control with precise temperature regulation.
According to the primary methodology, the annealing process occurs at 200°C. The furnace maintains this temperature uniformly while the nitrogen flow protects the sample, a combination that open-air heating methods cannot achieve.
Stability During Annealing
The enclosure of the Tube Furnace allows for a consistent flow dynamic.
Unlike a static oven, the continuous displacement ensures that any volatile byproducts released during the early stages of heating are swept away. This prevents them from re-depositing on the catalyst surface or interfering with the formation of the Ru-1 sites.
Understanding the Trade-offs
While nitrogen displacement is vital, it introduces specific operational constraints that must be managed to avoid failure.
The Risk of Incomplete Purging
If the displacement step is rushed, pockets of oxygen may remain in the "dead zones" of the tube.
Even trace amounts of oxygen can trigger agglomeration, causing the ruthenium to form large clusters. This significantly reduces the active surface area of the catalyst, rendering the delicate Ru-1 architecture useless.
Leak Integrity Reliance
The effectiveness of this step is entirely dependent on the seal integrity of the Tube Furnace.
If the furnace seals are compromised, the nitrogen flow cannot maintain positive pressure against the outside atmosphere. This creates a false sense of security where the operator believes the environment is inert, but micro-leaks are actively degrading the catalyst during the annealing phase.
Making the Right Choice for Your Goal
To maximize the performance of your Ru-1 catalysts, you must align your operational protocols with the sensitivity of the material.
- If your primary focus is Maximum Catalytic Activity: Prioritize a prolonged pre-heat purge duration to guarantee that oxygen levels are negligible before the temperature ramp begins.
- If your primary focus is Structural Reproducibility: rigorous leak-testing of the Tube Furnace seals is required to ensure the inert environment remains stable throughout the entire 200°C dwell time.
The difference between a high-performance single-atom catalyst and a failed batch often lies strictly in the thoroughness of the initial nitrogen purge.
Summary Table:
| Feature | Impact on Ru-1 Catalyst Quality |
|---|---|
| Atmosphere Control | Removes oxygen to prevent irreversible material degradation and oxidation. |
| Phase Stability | Ensures ruthenium remains in an atomically dispersed state (Ru-1) vs. clusters. |
| Thermal Precision | Enables uniform 200°C annealing while maintaining a strictly inert environment. |
| Byproduct Removal | Continuous gas flow sweeps away volatiles to prevent surface contamination. |
| Operational Integrity | Requires high-quality furnace seals to prevent micro-leaks and agglomeration. |
Elevate Your Material Synthesis with KINTEK Precision
Don't let oxygen contamination compromise your Ru-1 catalyst research. KINTEK’s advanced Tube Furnaces provide the airtight seal integrity and precise atmospheric control necessary for successful nitrogen displacement and atomic dispersion.
Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need standard lab equipment or a fully customizable high-temperature furnace, our solutions are engineered to meet your unique research requirements.
Ready to ensure the structural integrity of your next batch?
Contact KINTEK Today to Find Your Ideal Solution
Visual Guide
References
- DeSheng Su, Liang Chen. Efficient amine-assisted CO2 hydrogenation to methanol co-catalyzed by metallic and oxidized sites within ruthenium clusters. DOI: 10.1038/s41467-025-55837-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the purpose of flushing a tube furnace with high-purity argon for hours? Ensure Pure Silicon Steel Results
- What design features make horizontal furnaces versatile? Achieve High-Volume, Uniform Thermal Processing
- What are the benefits of a vertical tube furnace? Maximize Space and Purity in Your Lab
- What is a tubular furnace? Precision Heating for Lab and Industrial Applications
- What is the function of a high-purity quartz tube in the CVT synthesis of Fe3GeTe2? Expert Growth Insights
- How is sealing and atmosphere control achieved in a tube furnace? Master Precise Gas Environments for Your Lab
- How does the temperature controller function in a 70mm tube furnace? Achieve Precise Thermal Control for Your Lab
- Why are vacuum-sealed quartz tubes essential for Bi-Sb-Te phase diagrams? Ensure Chemical Fidelity in Your Alloy Synthesis



















