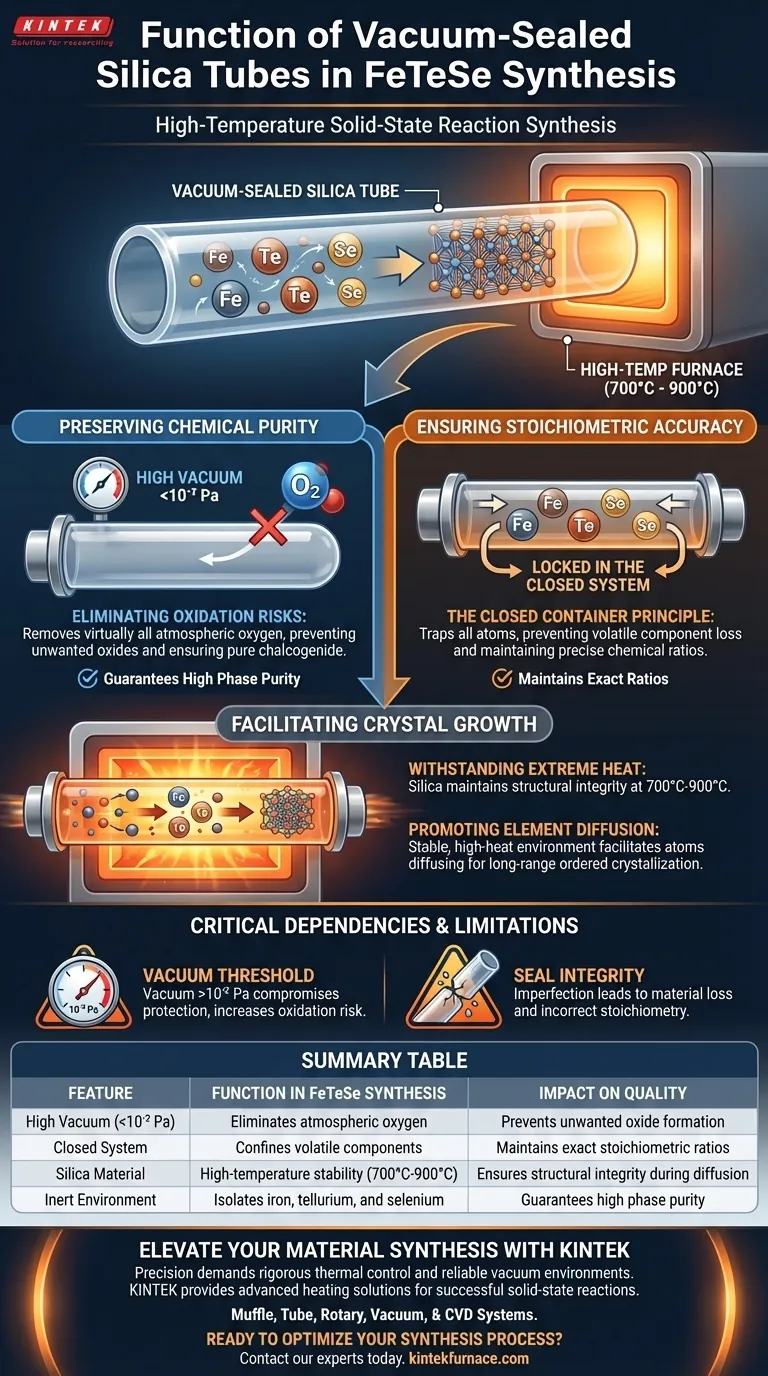
Vacuum-sealed silica tubes perform two essential functions in FeTeSe synthesis: isolating the reactants to prevent oxidation and strictly confining materials to maintain precise chemical ratios. By establishing a high-vacuum environment (less than 10⁻² Pa), these tubes protect iron, tellurium, and selenium from reacting with air while ensuring that volatile components do not escape during the 700°C to 900°C heating process.
Core Takeaway The success of solid-state synthesis lies in controlling the reaction environment. The silica tube acts as an inert pressure vessel that allows for the high temperatures necessary for element diffusion while physically preventing the contamination or material loss that would compromise the crystal's stoichiometry.

Preserving Chemical Purity
Eliminating Oxidation Risks
The primary threat to FeTeSe synthesis is the presence of oxygen. Iron, tellurium, and selenium are highly susceptible to oxidation when heated.
The Role of High Vacuum
By reducing the pressure to less than 10⁻² Pa, the silica tube removes virtually all atmospheric oxygen. This ensures that the final product remains a pure chalcogenide rather than degrading into unwanted oxides.
Ensuring Stoichiometric Accuracy
The Closed Container Principle
High-temperature reactions often lead to the volatilization of elements. If reactants escape as gas, the final chemical ratio (stoichiometry) will be incorrect.
Locking in the Ratio
The vacuum-sealed tube functions as a closed system. It traps all atoms inside the reaction zone, ensuring that the ratio of reactants you weigh at the start is exactly what reacts to form the crystal.
Facilitating Crystal Growth
Withstanding Extreme Heat
The synthesis requires a temperature range between 700°C and 900°C. Silica is chosen because it maintains structural integrity and chemical inertness at these extreme temperatures.
Promoting Element Diffusion
Solid-state reactions rely on atoms physically moving (diffusing) into one another to form a new structure. The stable, high-heat environment provided by the tube facilitates this diffusion, leading to long-range ordered crystallization among the components.
Critical Dependencies and Limitations
The Vacuum Threshold
The effectiveness of this method is binary. If the vacuum level rises above 10⁻² Pa, the protection is compromised, and oxidation becomes likely.
Seal Integrity
The "closed container" benefit relies entirely on a perfect seal. Any micro-cracks or imperfect seals will lead to material loss, immediately skewing the reactant ratios and ruining the crystal structure.
Making the Right Choice for Your Synthesis
To maximize the quality of your FeTeSe crystals, consider the specific requirements of your experimental goals:
- If your primary focus is phase purity: Ensure your vacuum pump can consistently achieve pressures significantly lower than 10⁻² Pa to eliminate all traces of oxygen.
- If your primary focus is structural consistency: Prioritize the integrity of the silica seal and precise temperature control (700°C–900°C) to guarantee the reactant ratios remain fixed during diffusion.
The vacuum-sealed silica tube is not just a container; it is an active control mechanism that defines the purity and structure of your final material.
Summary Table:
| Feature | Function in FeTeSe Synthesis | Impact on Quality |
|---|---|---|
| High Vacuum (<10⁻² Pa) | Eliminates atmospheric oxygen | Prevents unwanted oxide formation |
| Closed System | Confines volatile components | Maintains exact stoichiometric ratios |
| Silica Material | High-temperature stability (700°C-900°C) | Ensures structural integrity during diffusion |
| Inert Environment | Isolates iron, tellurium, and selenium | Guarantees high phase purity |
Elevate Your Material Synthesis with KINTEK
Precision in FeTeSe crystal growth demands more than just a tube; it requires rigorous thermal control and a reliable vacuum environment. KINTEK provides the advanced heating solutions necessary for successful high-temperature solid-state reactions.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temp furnaces are fully customizable to meet your unique experimental parameters, ensuring your research achieves the highest levels of purity and structural consistency.
Ready to optimize your synthesis process? Contact our experts today to find the perfect furnace for your laboratory needs.
Visual Guide
References
- Jiawei Liu, Qingyu Yan. Reaction-driven formation of anisotropic strains in FeTeSe nanosheets boosts low-concentration nitrate reduction to ammonia. DOI: 10.1038/s41467-025-58940-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why is controlling the residence time within a tube furnace critical for the synthesis of amorphous NiFe2O4 catalysts?
- What role does a high-temperature tube furnace play in the solid-state synthesis of LIB cathode materials? Key Insights
- In what scenarios are laboratory high-temperature tube furnaces or muffle furnaces utilized? Study MgTiO3-CaTiO3 Ceramics
- What is the process for using a vacuum tube experimental furnace? Master Precise Control for Your Lab
- How does a tube furnace contribute to the ammonia reduction annealing process for (NiZnMg)MoN catalysts? Optimize Phase Transitions
- Why is a high-temperature tube furnace utilized for the pyrolysis of Sr2TiO4 precursor powders? Achieving High Purity
- Why is a high-vacuum sealed quartz tube used in CVT? Ensuring High-Purity Fe4GeTe2 Single Crystal Growth
- What are the main operational considerations when using a lab tube furnace? Ensure Precision and Safety in Your Experiments



















