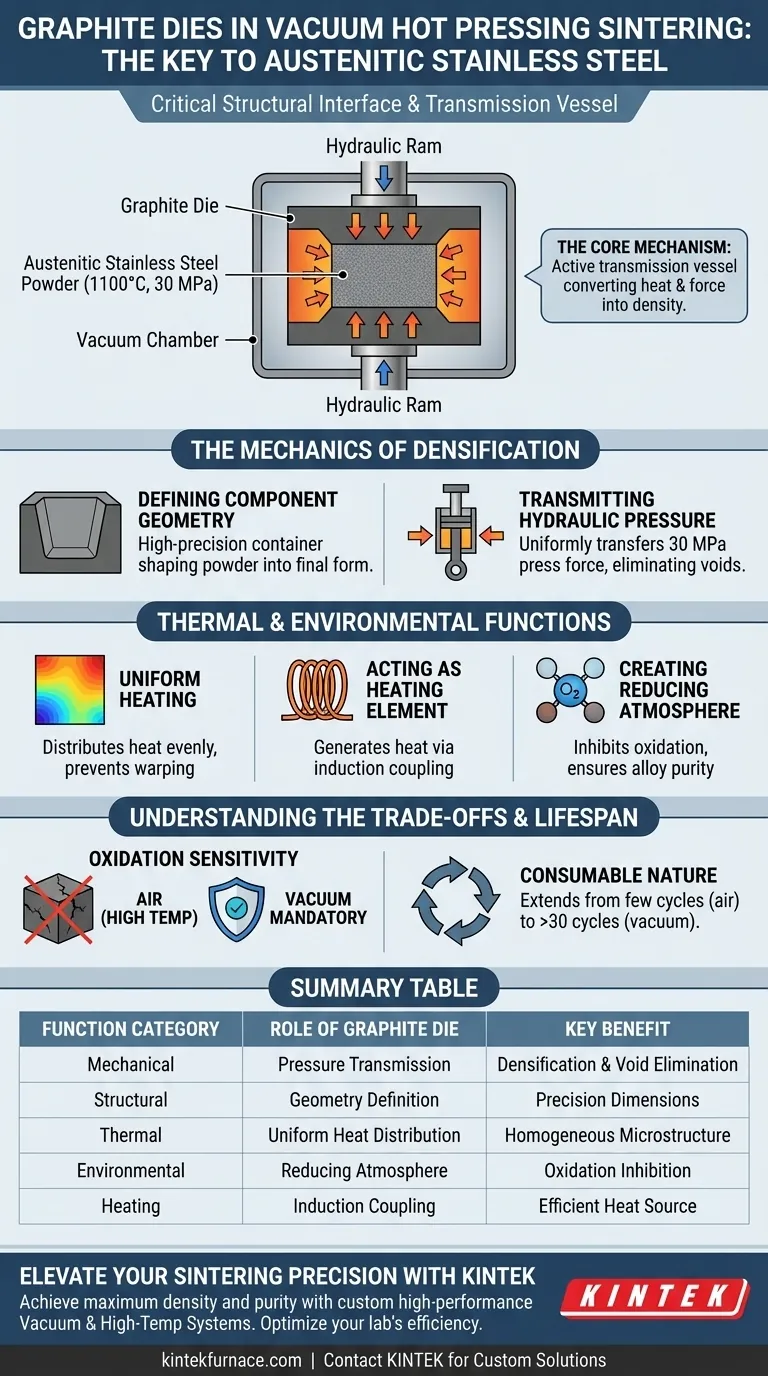
Graphite dies function as the critical structural interface during the vacuum hot pressing of austenitic stainless steel. Their primary role is twofold: they define the macroscopic geometry of the sintered component and serve as the medium for transmitting hydraulic pressure to the powder. This facilitates the densification of particles at high temperatures, typically around 1100°C.
The Core Mechanism The graphite die is not merely a passive container; it acts as an active transmission vessel that converts heat and axial force into material density. It allows for the simultaneous application of pressure (up to 30 MPa) and temperature, forcing the stainless steel powder to undergo plastic deformation and bond into a solid, high-strength structure.

The Mechanics of Densification
Defining Component Geometry
The most immediate function of the graphite die is shaping. It acts as a high-precision container that holds the loose austenitic stainless steel powder. Because the powder does not have a fixed form initially, the die defines the final dimensions and limits of the sintered sample, such as a cylindrical shape.
Transmitting Hydraulic Pressure
For sintering to occur effectively, the powder must be compressed. The graphite die withstands the immense axial force generated by the hydraulic press. It acts as the transfer medium, ensuring that this mechanical pressure is passed uniformly from the press rams to the powder body, which is essential for eliminating voids and achieving high density.
Thermal and Environmental Functions
Facilitating Uniform Heating
Graphite possesses excellent thermal conductivity. During the hot pressing cycle, the die ensures that heat is distributed evenly throughout the stainless steel powder. This uniformity is critical for preventing thermal gradients that could lead to warped components or uneven microstructures within the steel.
Acting as a Heating Element
In systems utilizing induction heating, the graphite die often serves as the heat source itself. Due to its electrical conductivity, the graphite couples with the induction field to generate heat, which is then transferred to the non-conductive or less conductive powder contents.
Creating a Reducing Atmosphere
At elevated temperatures, graphite can create a local reducing environment. This helps inhibit the oxidation of the material inside the die. For stainless steel, maintaining a low-oxygen environment is vital to preserve the purity of the alloy and ensure proper particle bonding.
Understanding the Trade-offs
High-Temperature Oxidation Sensitivity
While graphite is robust in a vacuum, it is highly susceptible to oxidation in air at high temperatures. The vacuum environment is therefore mandatory not only for the stainless steel but to protect the die itself. If exposed to oxygen while hot, the die would degrade rapidly, losing its dimensional accuracy.
Consumable Nature and Wear
Despite their high-temperature strength, graphite dies are considered consumables. They are subjected to significant mechanical stress and thermal cycling, which eventually limits their lifespan. However, using them in a vacuum significantly extends their utility—often from a few cycles in air to over 30 cycles in a vacuum—thereby reducing long-term material costs.
Making the Right Choice for Your Goal
To maximize the effectiveness of graphite dies in your sintering process, consider the following regarding your specific objectives:
- If your primary focus is Dimensional Precision: Ensure your vacuum system maintains a high integrity to prevent oxidation-induced erosion of the die walls, which alters part tolerance.
- If your primary focus is Material Purity: Leverage the graphite die's ability to create a reducing atmosphere, but verify that the process temperature (e.g., 1100°C) is strictly controlled to prevent adverse reactions.
Ultimately, the graphite die is the vessel that enables the transition from loose powder to a high-performance austenitic stainless steel component through the precise application of heat and pressure.
Summary Table:
| Function Category | Role of Graphite Die | Key Benefit for Stainless Steel |
|---|---|---|
| Mechanical | Pressure Transmission | Facilitates densification and eliminates voids up to 30 MPa |
| Structural | Geometry Definition | Ensures high-precision dimensions and final component shape |
| Thermal | Uniform Heat Distribution | Prevents thermal gradients and ensures uniform microstructure |
| Environmental | Local Reducing Atmosphere | Inhibits alloy oxidation and promotes superior particle bonding |
| Heating | Induction Coupling | Acts as a heat source for efficient, high-temperature processing |
Elevate Your Sintering Precision with KINTEK
Are you looking to achieve maximum density and purity in your high-performance alloys? Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to your unique sintering needs.
Our advanced thermal solutions ensure the perfect environment for your graphite dies, extending their lifespan and guaranteeing the integrity of your austenitic stainless steel components.
Ready to optimize your lab's efficiency? Contact KINTEK today to discuss your custom furnace solution!
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- Why is a high vacuum required in a hot-pressing furnace? Achieve Perfect Transparency in Fluoride Ceramics
- What is the primary role of a vacuum hot press furnace? Synthesis of Ti-Al3Ti Laminated Composites Explained
- What key role does a vacuum hot pressing furnace play in ADSC alloys? Achieve Near-Theoretical Density & Purity
- What are the primary applications of vacuum press technology? Achieve Superior Material Bonding and Shaping
- What are the size variations available for hot press furnaces? Choose the Right Size for Your Lab or Production Needs
- What is the impact of grain structure on material properties in hot pressing vs. cold compacting and sintering? Optimize Your Powder Metallurgy Process
- How are vacuum hot pressing sintering furnaces classified based on their operating temperature? A Guide to Low, Medium, and High-Temp Ranges
- What role does a hot press sintering furnace play in Cf-UHTC production? Achieve Peak Density in Refractory Composites



















