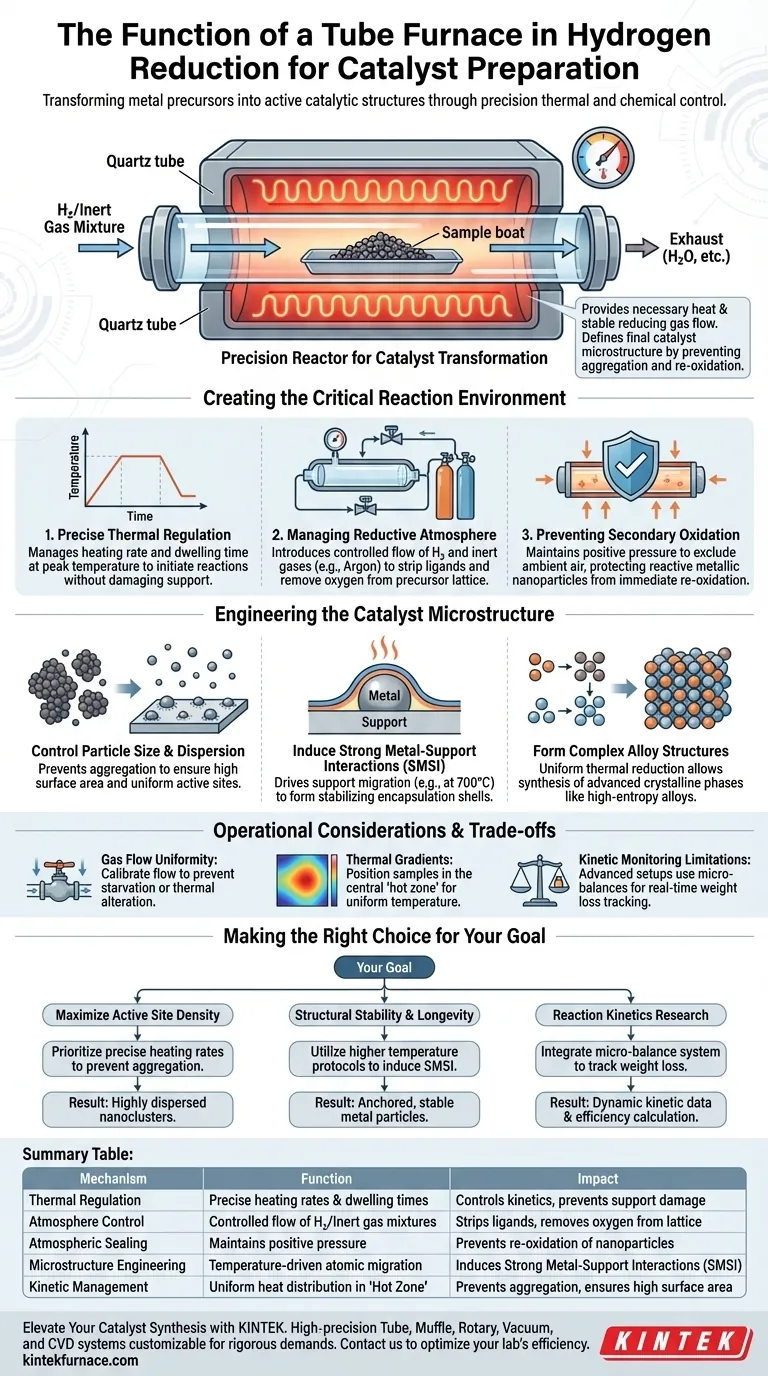
A tube furnace functions as a precision reactor designed to transform metal precursors into active catalytic structures through a strictly controlled thermal and chemical environment. During the hydrogen reduction phase, it provides the necessary heat to drive chemical reduction while maintaining a stable flow of reducing gases, such as a hydrogen-argon mixture, to ensure the precursors are fully converted into active metal nanoparticles.
The primary value of the tube furnace lies in its ability to define the catalyst's final microstructure. By strictly regulating temperature profiles and gas composition, it ensures the uniform formation of metallic active sites while preventing the aggregation or re-oxidation that would degrade catalytic performance.

Creating the Critical Reaction Environment
To achieve high-performance catalysts, the reduction environment must be isolated and manipulated with extreme precision. The tube furnace accomplishes this through three specific mechanisms.
Precise Thermal Regulation
The furnace manages the heating rate and "dwelling time" (the duration at peak temperature). This control is essential because different reduction reactions require specific energy thresholds to initiate without damaging the support material.
Managing the Reductive Atmosphere
The furnace utilizes a sealed tube to introduce a controlled flow of reducing gases, typically a mixture of hydrogen and inert gases like argon or nitrogen. This specific atmosphere strips ligands from metal precursors and removes oxygen atoms from the lattice structure.
Preventing Secondary Oxidation
By maintaining a positive pressure of reducing gas within the sealed tube, the furnace prevents ambient air from entering the reaction zone. This protection is critical at high temperatures, where newly formed metallic particles are highly reactive and prone to immediate re-oxidation.
Engineering the Catalyst Microstructure
Beyond simply heating the sample, the tube furnace acts as a tool for "microstructural engineering." The parameters set during this phase directly dictate the physical arrangement of atoms on the catalyst surface.
Controlling Particle Size and Dispersion
The furnace facilitates the transformation of oxidized precursors into highly dispersed metallic nanoclusters. By controlling the reduction kinetics, the process prevents the metal atoms from clumping together (aggregation), ensuring a high surface area for future reactions.
Inducing Strong Metal-Support Interactions (SMSI)
At higher temperatures (e.g., 700 °C), the thermal energy provided by the furnace can drive the migration of support materials onto the metal surface. This creates an encapsulation shell or "Strong Metal-Support Interaction," which stabilizes the metal particles and modifies their electronic properties for specific reactions.
Forming Complex Alloy Structures
precise temperature control (e.g., holding strictly at 350 °C) allows for the synthesis of complex materials, such as high-entropy alloys with single-phase structures. The furnace ensures the thermal reduction is uniform enough to create these advanced crystalline phases, which are essential for specialized applications like the hydrogen evolution reaction (HER).
Operational Considerations and Trade-offs
While the tube furnace is the standard for precision reduction, operators must be aware of specific constraints to ensure data integrity and safety.
Gas Flow Uniformity
The flow rate of the hydrogen mixture must be carefully calibrated to the tube diameter and sample volume. Inadequate flow can lead to "starvation" zones where reduction is incomplete, while excessive flow may alter the thermal profile of the reaction zone.
Thermal Gradients
Although tube furnaces offer excellent stability, slight temperature gradients can exist along the length of the tube. Samples must be positioned in the "hot zone"—the central region of the tube where the temperature is verified to be uniform—to guarantee consistent results.
Kinetic Monitoring Limitations
Standard tube furnaces are "black boxes" regarding real-time reaction progress. However, advanced setups integrate electronic micro-balances to monitor weight loss in real-time. Without this integration, operators rely on post-process analysis rather than dynamic kinetic data.
Making the Right Choice for Your Goal
The configuration of your reduction phase depends heavily on the specific catalytic properties you aim to develop.
- If your primary focus is maximizing active site density: Prioritize precise heating rates to prevent particle aggregation, ensuring metal precursors transform into highly dispersed nanoclusters.
- If your primary focus is structural stability and longevity: Utilize higher temperature protocols to induce Strong Metal-Support Interactions (SMSI), which anchor metal particles and prevent sintering during use.
- If your primary focus is reaction kinetics research: Integrate a micro-balance system to track real-time weight loss, allowing you to calculate reduction efficiency and reaction rates dynamically.
Ultimately, the tube furnace is not just a heating element; it is the instrument that defines the geometry, stability, and efficiency of your final catalyst.
Summary Table:
| Mechanism | Function in Hydrogen Reduction | Impact on Catalyst |
|---|---|---|
| Thermal Regulation | Precise heating rates & dwelling times | Controls reduction kinetics & prevents support damage |
| Atmosphere Control | Controlled flow of H₂/Inert gas mixtures | Strips ligands & removes oxygen from lattice structures |
| Atmospheric Sealing | Maintains positive pressure/Oxygen exclusion | Prevents re-oxidation of reactive metallic nanoparticles |
| Microstructure Engineering | Temperature-driven atomic migration | Induces Strong Metal-Support Interactions (SMSI) |
| Kinetic Management | Uniform heat distribution in 'Hot Zone' | Prevents aggregation and ensures high surface area |
Elevate Your Catalyst Synthesis with KINTEK
Precision is the difference between a failed precursor and a high-performance active catalyst. Backed by expert R&D and world-class manufacturing, KINTEK offers high-precision Tube, Muffle, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of hydrogen reduction and material science.
Our lab high-temperature furnaces are fully customizable to your unique thermal profiles and gas flow requirements, ensuring uniform particle dispersion and stable SMSI formation. Contact us today to optimize your lab's efficiency and discover how our specialized heating solutions can transform your research outcomes.
Visual Guide
References
- Lu Chen, Feng Ryan Wang. Tuning the selectivity of NH3 oxidation via cooperative electronic interactions between platinum and copper sites. DOI: 10.1038/s41467-024-54820-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is the first stage of sintering in a tube vacuum sintering furnace necessary? Master the Space-Holder Technique
- What optional features are available for tube furnaces? Enhance Your Materials Processing with Precision Control
- How does a high vacuum tube furnace contribute to the carbonization process? Engineered Hard Carbon Synthesis
- What is the maximum temperature for a tube furnace? Unlock the Right Heat for Your Application
- How do high-temperature laboratory tube furnaces ensure environmental stability? Precision Thermal Reduction Tips
- Why is an industrial-grade high-temperature tube furnace used for TiO2NW? Optimize Nanowire Annealing
- What is the role of a tube sintering furnace during the activation of carbon materials? Expert Guide to CO2 Activation
- What factors should be considered when choosing a tube furnace for a lab? Ensure Precision and Safety in Your Experiments



















