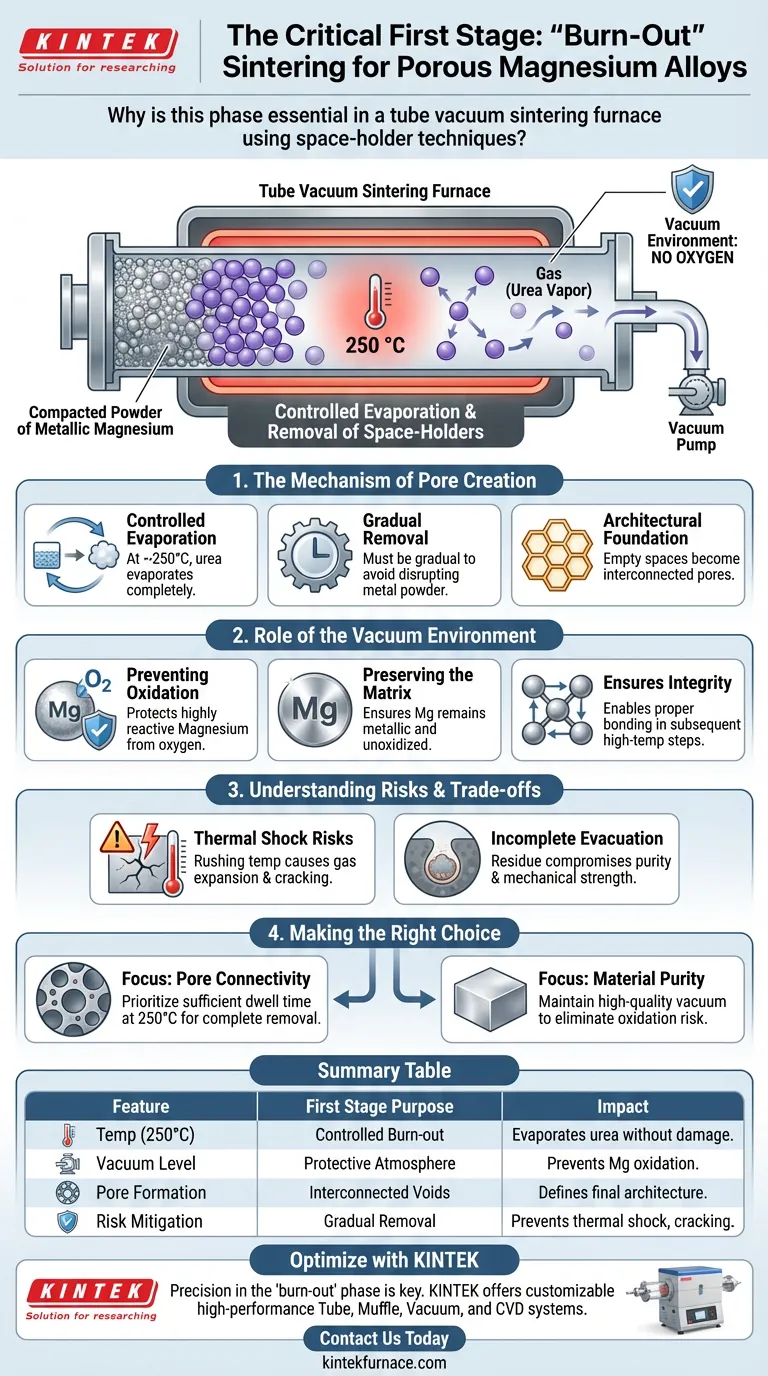
The first stage of sintering is the critical "burn-out" phase essential for structural formation. It is specifically designed to remove space-holding agents, such as urea, through controlled evaporation at lower temperatures like 250 °C. This creates the necessary interconnected pore structure while employing a vacuum to protect the highly reactive magnesium matrix from severe oxidation.
By effectively separating pore formation from the final metal bonding, this stage prevents the magnesium from oxidizing while ensuring the space-holder is completely evacuated.

The Mechanism of Pore Creation
Controlled Evaporation
The primary goal of this initial stage is to facilitate the phase change of the space-holder. At temperatures around 250 °C, agents like urea evaporate.
Gradual Removal
This process must be controlled and gradual. If the agent evaporates too quickly, it can disrupt the surrounding metal powder.
Architectural Foundation
As the urea leaves the system, it creates specific voids. These empty spaces become the interconnected pores that define the material's porous characteristics.
The Role of the Vacuum Environment
Preventing Oxidation
Magnesium is an extremely reactive metal, particularly when heated. Without a protective environment, it would react aggressively with oxygen.
Preserving the Matrix
The vacuum environment is non-negotiable during this phase. It ensures that while the urea is being removed, the magnesium powder remains metallic and unoxidized.
Ensuring Structural Integrity
If oxidation were to occur at this stage, the magnesium particles would develop oxide layers. These layers would prevent proper bonding during the subsequent high-temperature sintering.
Understanding the Risks and Trade-offs
Thermal Shock Risks
This stage requires strict temperature regulation. Rushing to higher temperatures before the space-holder is fully removed can cause rapid gas expansion, cracking the delicate material.
Incomplete Evacuation
If the vacuum pressure is insufficient or the time at 250 °C is too short, residue from the space-holder may remain. This contamination compromises the purity and mechanical strength of the final alloy.
Making the Right Choice for Your Goal
To ensure the success of your porous magnesium alloy project, prioritize the specific parameters of this first stage.
- If your primary focus is Pore Connectivity: Ensure the dwell time at 250 °C is sufficient to allow for the complete and gentle evacuation of the entire urea content.
- If your primary focus is Material Purity: Maintain a high-quality vacuum throughout the entire evaporation phase to eliminate any risk of magnesium oxidation.
Mastering this initial low-temperature phase is the only way to guarantee a porous structure that is both mechanically stable and chemically pure.
Summary Table:
| Feature | First Stage Purpose | Impact on Porous Magnesium |
|---|---|---|
| Temperature (250°C) | Controlled Burn-out | Evaporates urea/space-holders without damaging structure. |
| Vacuum Level | Protective Atmosphere | Prevents oxidation of reactive magnesium powder. |
| Pore Formation | Interconnected Voids | Defines the final material's architectural foundation. |
| Risk Mitigation | Gradual Removal | Prevents thermal shock, gas expansion, and cracking. |
Optimize Your Advanced Material Synthesis with KINTEK
Precision in the 'burn-out' phase is the difference between a high-performance porous alloy and a failed project. KINTEK provides the specialized equipment needed to master these delicate thermal cycles. Backed by expert R&D and manufacturing, we offer high-performance Tube, Muffle, Vacuum, and CVD systems—all fully customizable to meet your specific research or production requirements.
Don't let oxidation or thermal shock compromise your structural integrity. Contact us today to discover how our high-temperature lab furnaces can enhance your material purity and sintering efficiency.
Visual Guide
References
- Divyanshu Aggarwal, Manoj Gupta. Porous Mg–Hydroxyapatite Composite Incorporated with Aloe barbadensis Miller for Scaphoid Fracture Fixation: A Natural Drug Loaded Orthopedic Implant. DOI: 10.3390/app14041512
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the function of a tube furnace and nitrogen flow in biomass carbonization? Unlock Superior Bio-Carbon Quality
- Why is a tube high-temperature furnace required for Au@MoSe2/graphene composites? Precision Reaction Control
- What are the core advantages of using a Drop Tube Furnace compared to a TGA? Bridge Lab Theory and Industrial Reality
- What is the key component of a tube furnace and how is it constructed? Unlock Precision Heating for Your Lab
- What role does a high-temperature tube furnace play in POLO contact structures? Unlock High-Efficiency Silicon Contacts
- What physical conditions does a tube furnace provide for core-shell catalysts? Precision Reduction & SMSI Control
- What is the significance of using a high-temperature tube furnace for thermal annealing? Optimize hDMHA Electrodes
- How does a vacuum tube nitriding system control the reaction environment? Precision Surface Hardening for AISI 304



















