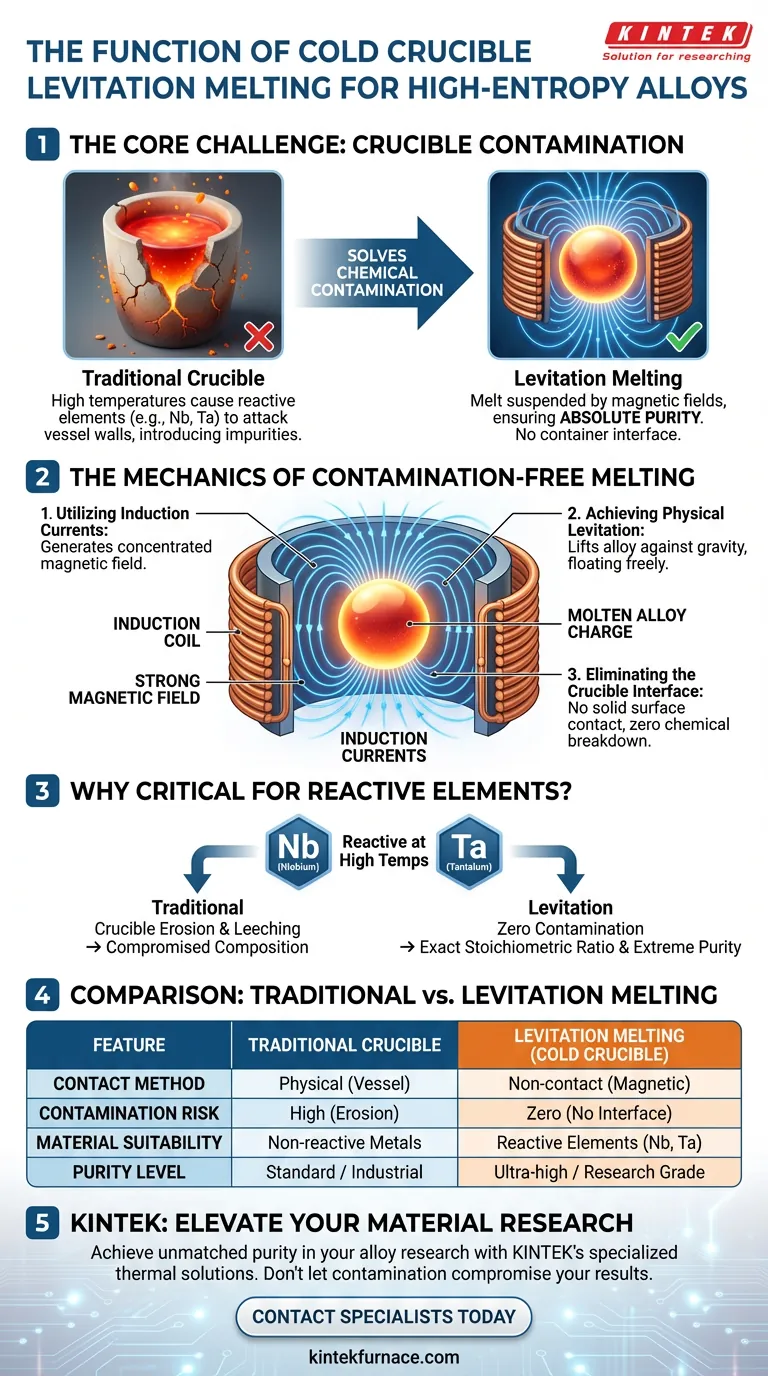
The primary function of a cold crucible or levitation melting furnace is to melt metal without physical contact to ensure absolute purity. By utilizing induction currents to generate strong magnetic fields, the furnace physically levitates the alloy charge. This isolation effectively eliminates the risk of the molten material reacting with a containment vessel, which is a critical requirement when processing high-entropy alloys containing reactive elements.
Core Takeaway Levitation melting solves the problem of chemical contamination that plagues traditional alloy processing. By suspending the melt using magnetic fields, this method eliminates the need for a physical crucible, ensuring that high-melting-point, reactive elements like niobium and tantalum remain free from foreign impurities.
The Mechanics of Contamination-Free Melting
Utilizing Induction Currents
The furnace operates by directing powerful induction currents through a coil surrounding the processing area. These currents do not merely heat the material; they generate a concentrated, high-intensity magnetic field.
Achieving Physical Levitation
This magnetic field exerts a physical force on the metallic charge, lifting it against gravity. Consequently, the alloy is suspended in mid-air, melting solely through induction energy while floating freely within the coil.
Eliminating the Crucible Interface
The defining feature of this process is the absence of a traditional containment vessel. Because the melt never touches a solid surface, there is no interface where chemical breakdown or diffusion can occur.
Why High-Entropy Alloys Require This Method
Handling Reactive Elements
High-entropy alloys often incorporate "reactive" elements, such as niobium and tantalum. These materials are chemically aggressive at high temperatures and will readily bond with standard crucible materials like ceramics or graphite.
Preventing Material Leeching
If a traditional crucible were used, these reactive elements would attack the vessel walls. This reaction would erode the crucible and introduce foreign atoms into the mix, compromising the alloy's chemical composition.
Ensuring Extreme Purity
For high-performance applications, maintaining the exact stoichiometric ratio of the alloy is vital. Cold crucible levitation is essential for these specific mixtures because it guarantees that the final product contains only the intended elements, with zero contamination from processing equipment.
Understanding the Operational Trade-offs
Specificity vs. Simplicity
While effective, this method is a specialized solution designed for a specific set of problems. It is inherently more complex than standard vacuum arc melting or induction melting in a ceramic pot.
Energy and Stability
The process relies entirely on the precise application of magnetic fields to maintain suspension. It is strictly necessary only when the cost of complexity is outweighed by the absolute need for purity in reactive, high-melting-point formulations.
Making the Right Choice for Your Goal
When deciding on a melting process for complex alloy preparation, consider your material constraints:
- If your primary focus is extreme purity: You must use levitation melting to prevent trace contamination from refractory materials.
- If your primary focus is processing reactive elements (Nb, Ta): You need this method to prevent the melt from chemically attacking and destroying traditional crucibles.
This technology converts the challenge of containment into a matter of physics, allowing for the creation of pristine materials that would otherwise be impossible to manufacture.
Summary Table:
| Feature | Traditional Crucible Melting | Cold Crucible / Levitation Melting |
|---|---|---|
| Contact Method | Physical contact with vessel | Non-contact (Magnetic Levitation) |
| Contamination Risk | High (Crucible erosion/leeching) | Zero (No crucible interface) |
| Material Suitability | Non-reactive metals | Reactive elements (Nb, Ta, Ti) |
| Heating Principle | Thermal conduction/induction | Induction currents & magnetic fields |
| Purity Level | Standard / Industrial | Ultra-high / Research grade |
Achieve Unmatched Purity in Your Alloy Research
Maintaining stoichiometric integrity is critical when working with complex high-entropy alloys and reactive elements. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems—including customizable lab high-temp furnaces designed for your unique material needs.
Don't let crucible contamination compromise your results. Contact our specialists today to discover how KINTEK's precision thermal solutions can elevate your material preparation and ensure the absolute purity of your reactive formulations.
Visual Guide
References
- Laurent Peltier, Jérome Slowensky. Design of Multiphase Compositionally Complex Alloys for Enhanced Hardness at Elevated Temperatures and Machinability: Comparative Study with Inconel 718. DOI: 10.1002/adem.202501146
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the principle of a vacuum induction melting furnace based on? Achieve High-Purity Metal Melting
- Why can a high vacuum cause process failure in Nb-MASC alloys? Prevent Critical Loss of Volatile Elements
- What role does a vacuum arc melting furnace play in Ti-6Al-7Nb-xTa alloys? Precision Melting & Purity
- What is the primary purpose of preheating charge materials for C95800 aluminum bronze? Eliminate Porosity Defects
- How do graphite crucible furnaces improve processing times? Achieve Unmatched Speed and Uniformity
- What are the three main components of a vacuum induction melting furnace? Key Systems for Pure Metal Production
- What future applications might benefit from induction technology? Unlock Next-Gen Manufacturing & Green Energy
- What finishing processes follow metal casting in induction furnaces? Achieve Precision and Efficiency in Metalworking



















