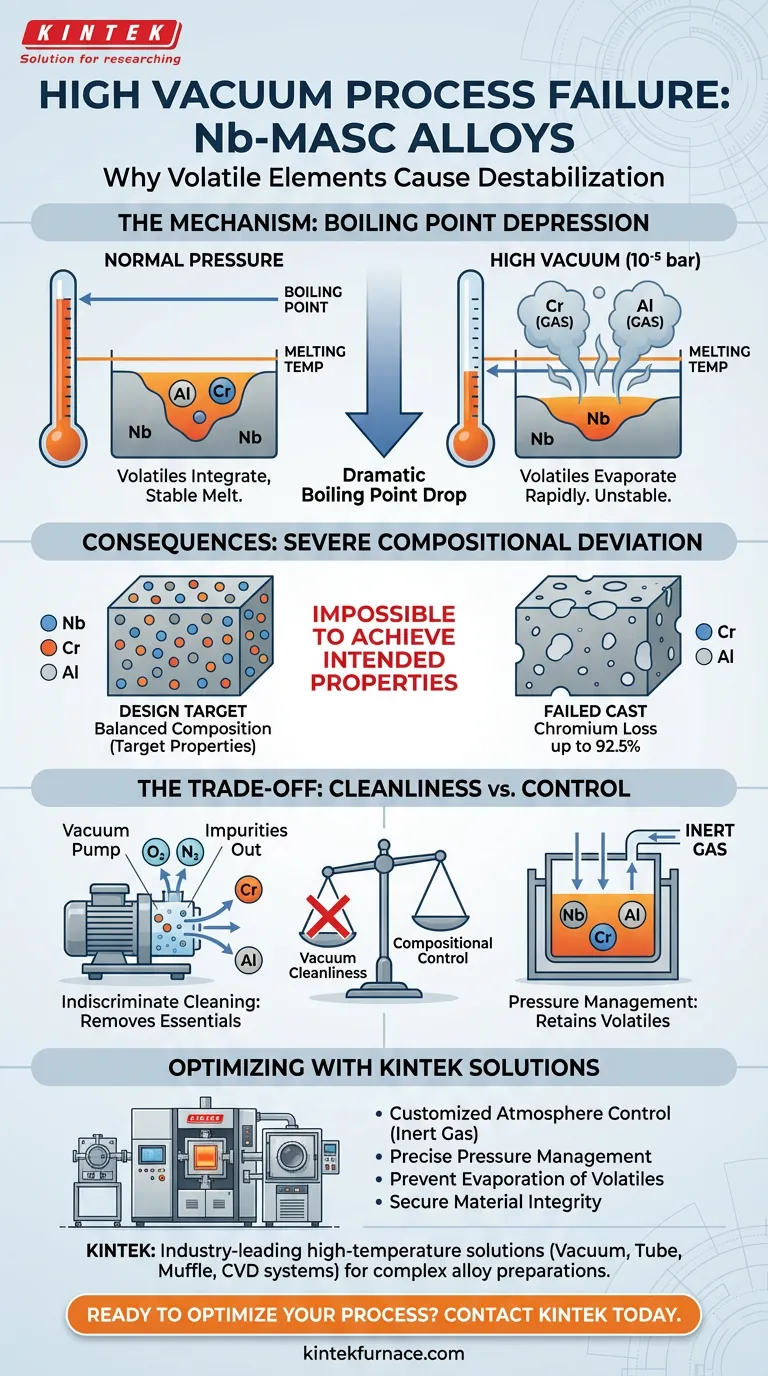
High vacuum environments fundamentally destabilize the melting process for Nb-MASC alloys by dramatically lowering the boiling points of volatile elements like aluminum (Al) and chromium (Cr). Because these depressed boiling points often fall below the temperature required to melt the overall alloy, these critical elements evaporate rapidly rather than integrating into the melt.
Core Takeaway Processing alloys with volatile components in a high vacuum causes massive evaporation due to boiling point depression. This results in severe chemical deviation from the design target—such as chromium loss up to 92.5%—making it impossible to achieve the intended material properties.
The Mechanism of Evaporation
Boiling Point Depression
In a high vacuum environment, such as 10^-5 bar, the thermodynamic properties of materials change significantly. Specifically, the temperature required for a substance to transition from liquid to gas (boiling point) decreases as the surrounding pressure drops.
The Melting Temperature Conflict
For Nb-MASC alloys, the processing temperature must be high enough to melt the refractory matrix (Niobium). However, under high vacuum, this necessary processing temperature exceeds the lowered boiling points of volatile additives like aluminum and chromium.
Instead of melting and mixing, these elements immediately vaporize.
Consequences for Alloy Integrity
Severe Compositional Deviation
The immediate result of this phenomenon is a drastic shift in chemical composition. The alloy loses its volatile components to the vacuum chamber atmosphere rather than retaining them in the casting.
Quantifying the Loss
The scale of this loss is often catastrophic for the material's design. Research indicates that chromium loss can reach as high as 92.5% under these conditions.
This degree of depletion means the final cast product bears little resemblance to the intended stoichiometric design.
Understanding the Trade-offs
Vacuum Cleanliness vs. Compositional Control
Vacuum melting is typically prized for its ability to remove impurities and prevent oxidation. However, when working with high-vapor-pressure elements (volatiles), this benefit becomes a liability.
The "Cleanliness" Trap
While a high vacuum might successfully remove oxygen or nitrogen, it acts indiscriminately, "cleaning" the alloy of its essential alloying elements as well.
You cannot prioritize atmospheric purity over vapor pressure management when low-melting-point elements are involved.
Optimizing the Furnace Atmosphere
To successfully prepare Nb-MASC alloys, you must abandon high vacuum protocols in favor of atmosphere control.
- If your primary focus is Compositional Accuracy: You must adjust the furnace atmosphere (likely introducing an inert gas) to raise the ambient pressure, thereby raising the boiling points of Al and Cr above the alloy's melting temperature.
- If your primary focus is Process Stability: Avoid high vacuum settings (e.g., 10^-5 bar) entirely during the melt phase to prevent the violent evaporation of volatile components.
Success in alloying volatile elements requires matching your furnace pressure to the vapor pressure limits of your most sensitive ingredients.
Summary Table:
| Element Involved | Role in Process | Impact of High Vacuum (10^-5 bar) | Consequence of Failure |
|---|---|---|---|
| Niobium (Nb) | Refractory Matrix | Requires high melting temperature | High heat triggers volatile evaporation |
| Chromium (Cr) | Volatile Additive | Boiling point drops below melting point | Up to 92.5% loss of total content |
| Aluminum (Al) | Volatile Additive | Rapid vaporization during melt phase | Severe compositional deviation |
| Inert Gas | Pressure Control | Not present in high vacuum | Essential to prevent vapor pressure loss |
Secure Your Material Integrity with KINTEK
Don't let volatile element loss compromise your research or production. KINTEK provides industry-leading high-temperature solutions—including Vacuum, Tube, Muffle, and CVD systems—specifically designed to handle complex alloy preparations.
Backed by expert R&D and precision manufacturing, our furnaces are fully customizable to provide the exact atmosphere control needed to prevent evaporation in sensitive materials like Nb-MASC.
Ready to optimize your alloying process? Contact us today to discuss your unique laboratory needs with our technical team.
Visual Guide
References
- M. Guglielmi, Sebastian Herbst. Induction melting in cold crucible furnace for the production of components in turbine applications. DOI: 10.22364/mhd.61.1-2.5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are the benefits of graphite crucible furnaces? Achieve Superior High-Temperature Processing
- How scalable are induction gold melting furnaces for different operations? From Jewelry to Industrial Refining
- What are the raw materials for induction furnace? The Essential Guide to Charge & Construction Materials
- What is the principle of induction heating? Master the Physics of Non-Contact Heating
- What are the key components of a modern induction melting furnace? A Guide to Core Systems & Performance
- Why is repeated remelting and ingot flipping required in a vacuum arc furnace when synthesizing Ti40Zr40Mo10W10 alloys?
- What is the function of a Vacuum Induction Melting Furnace? Essential Precision for Steel Research
- What are the key features of a medium frequency induction furnace? Unlock Faster, Cleaner Metal Processing



















