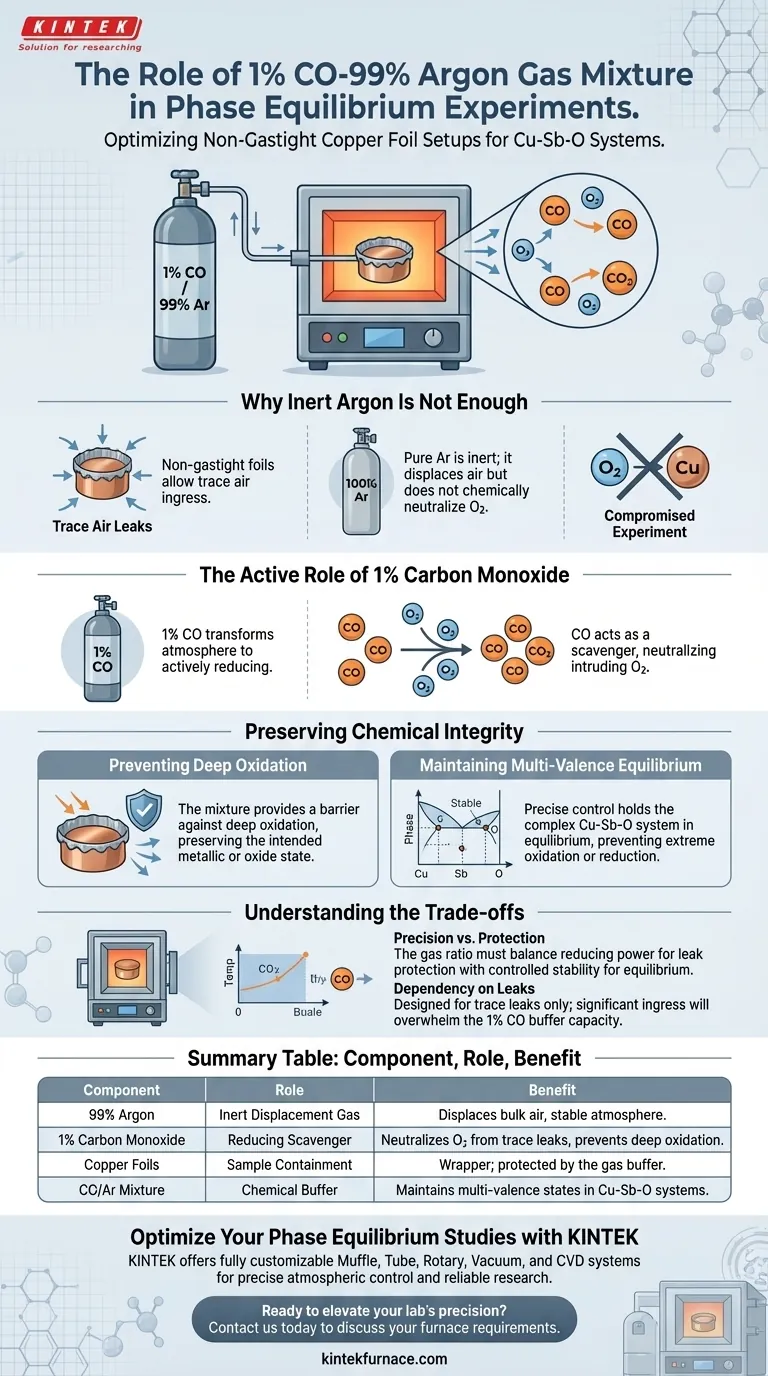
The primary function of a 1% CO-99% Argon gas mixture is to create a controlled reducing environment that actively protects copper samples during phase equilibrium experiments. In setups utilizing non-gastight copper foils, this atmosphere neutralizes oxygen introduced via trace air leaks. It ensures the preservation of the specific multi-valence state equilibrium required for accurate study of the copper-antimony-oxygen system.
This gas mixture acts as a chemical buffer, preventing unintended deep oxidation caused by system leaks while preserving the delicate valence states necessary for valid experimental results.

Why "Inert" Argon Is Not Enough
The Vulnerability of Non-Gastight Foils
In these experiments, copper foils serve as wrappers or containers for the sample, but they are not hermetically sealed.
Because the setup is "non-gastight," trace amounts of air can penetrate the physical barrier.
The Limitation of Pure Argon
Pure Argon is an inert gas; it displaces air but cannot chemically neutralize oxygen that leaks into the system.
If oxygen enters an environment of pure Argon, it remains free to react with the heated sample, compromising the experiment.
The Active Role of Carbon Monoxide
The addition of 1% Carbon Monoxide (CO) transforms the atmosphere from purely inert to actively reducing.
The CO acts as a scavenger, reacting with intruding oxygen to neutralize it before it can degrade the copper foils or the sample inside.
Preserving Chemical Integrity
Preventing Deep Oxidation
The primary risk in this setup is "unintended deep oxidation," where excess oxygen fundamentally alters the sample's composition.
The 1% CO mixture provides a sufficient barrier against this oxidation, ensuring the copper remains in its intended metallic or oxide state rather than being consumed by atmospheric oxygen.
Maintaining Multi-Valence Equilibrium
The copper-antimony-oxygen system is complex and relies on a specific balance of multi-valence states.
This precise atmospheric control is essential to hold the system in equilibrium, preventing the chemistry from shifting too far toward oxidation or reduction.
Understanding the Trade-offs
Precision vs. Protection
The effectiveness of this method relies on the specific ratio of the gas mixture.
The environment must be reducing enough to counteract air leaks, but controlled enough to maintain the specific equilibrium of the Cu-Sb-O system.
Dependency on Flow and Leaks
While the mixture offers protection, it is designed to handle trace leaks, not gross failure of the containment.
Reliance on this atmosphere assumes that air ingress is minimal; significant leaks would likely overwhelm the 1% CO buffer capacity.
Making the Right Choice for Your Experiment
To apply this to your own phase equilibrium studies, consider your specific constraints:
- If your primary focus is sample protection: Use this mixture to scavenge oxygen when using imperfect physical barriers like crimped foils.
- If your primary focus is data validity: Rely on this atmosphere to stabilize complex multi-valence systems that are sensitive to both oxidation and excessive reduction.
By balancing active protection with chemical stability, this specific gas mixture ensures reliable data even when physical containment is imperfect.
Summary Table:
| Component | Role in Experiment | Benefit to Phase Equilibrium |
|---|---|---|
| 99% Argon | Inert Displacement Gas | Displaces bulk air and provides stable atmosphere |
| 1% Carbon Monoxide | Reducing Scavenger | Neutralizes oxygen from trace leaks to prevent deep oxidation |
| Copper Foils | Sample Containment | Acts as a wrapper; protected by the gas buffer |
| CO/Ar Mixture | Chemical Buffer | Maintains multi-valence states in Cu-Sb-O systems |
Optimize Your Phase Equilibrium Studies with KINTEK
Precise atmospheric control is the backbone of reliable thermal analysis and phase equilibrium research. At KINTEK, we understand that even trace oxygen leaks can compromise complex multi-valence systems like the copper-antimony-oxygen system.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet your specific gas-mixing and atmospheric protection needs. Whether you are performing delicate metallurgical research or advanced materials synthesis, our high-temp lab furnaces provide the stability and control required for valid experimental results.
Ready to elevate your lab's precision? Contact us today to discuss your unique furnace requirements with our technical team.
Visual Guide
References
- Hamed Abdeyazdan, Evgueni Jak. Phase equilibria in the CuO <sub>0.5</sub> –SbO <sub>1.5</sub> –SiO <sub>2</sub> system. DOI: 10.1111/jace.70123
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- Why is a constant temperature drying oven used at 100 °C for HPC preparation? Ensure Optimal Carbonization Results
- Why is a furnace with programmed temperature control required for catalyst regeneration? Ensure Catalyst Stability
- Why specific constant temperature holding times for NbC and Cr7C3? Achieve Stoichiometric Precision in Lab Synthesis
- What is the main purpose of annealing? A Guide to Controlling Material Properties
- What role does precision analytical equipment play in petrochemical R&D? Engineering the Future of Efficient Refining
- What role does a high-pressure autoclave play in the synthesis of the (NiZnMg)MoN precursor? Achieve Structural Precision
- Why is a vacuum desiccator essential for studying geopolymer porosity? Achieve Precise Material Characterization
- How does direct technical consultation support the acquisition of customized high-temperature furnace systems? Expert R&D



















