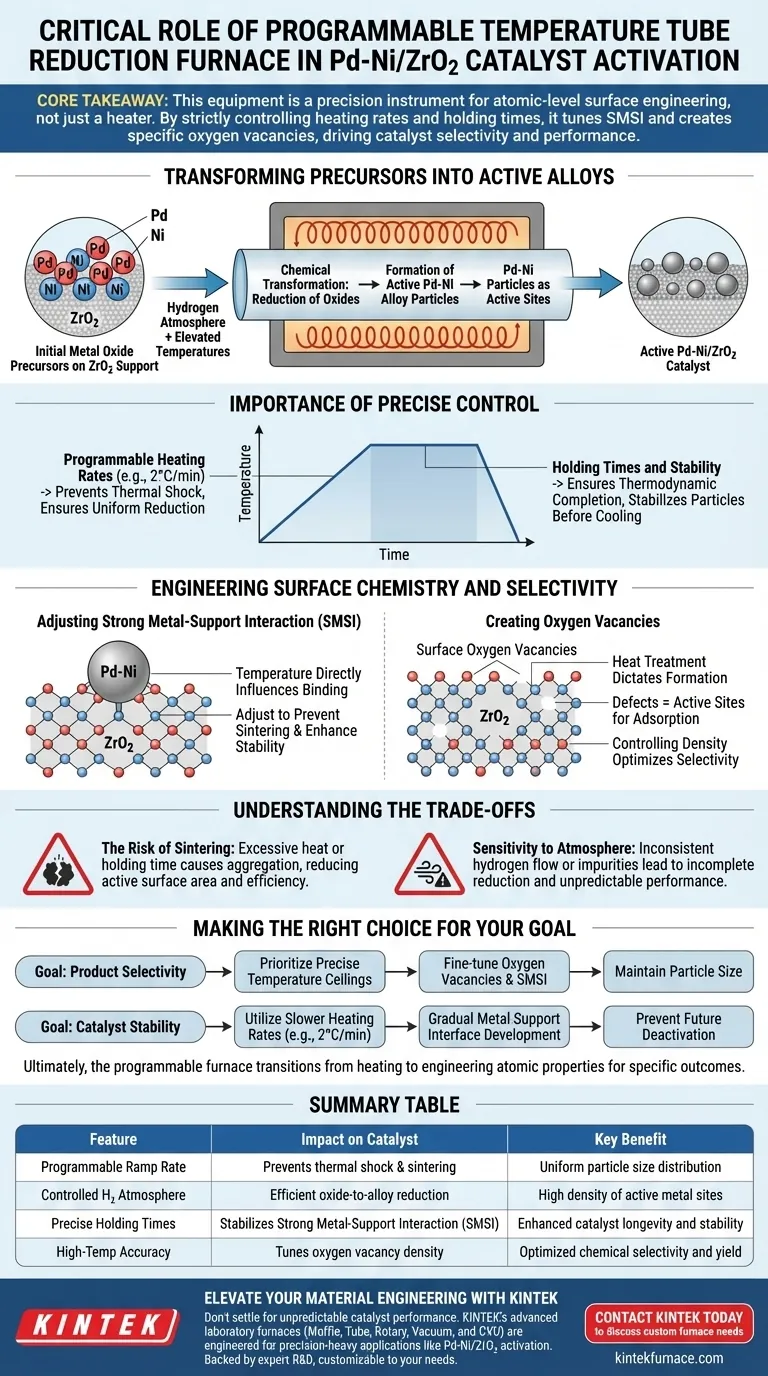
The programmable temperature tube reduction furnace serves as the definitive tool for activating Pd-Ni/ZrO2 catalysts. Its critical role is to precisely execute the high-temperature reduction of metal oxide precursors into active Palladium-Nickel (Pd-Ni) alloy particles under a controlled hydrogen atmosphere.
Core Takeaway: This equipment is not merely a heating device; it is a precision instrument for atomic-level surface engineering. By strictly controlling heating rates and holding times, the furnace tunes the Strong Metal-Support Interaction (SMSI) and creates specific oxygen vacancies, which are the primary drivers of the catalyst's final selectivity and performance.

Transforming Precursors into Active Alloys
The Reduction Mechanism
The primary function of the furnace is to facilitate a chemical transformation.
It exposes the catalyst material to a hydrogen atmosphere at elevated temperatures.
This environment reduces the initial metal oxide precursors found on the support material.
Formation of Pd-Ni Particles
The ultimate goal of this thermal treatment is the creation of specific metallic structures.
Through reduction, the furnace converts the oxides into active Pd-Ni alloy particles.
These particles serve as the active sites where future catalytic reactions will occur.
The Importance of Precise Control
Programmable Heating Rates
The "programmable" aspect of the furnace is vital for catalyst quality.
It allows for exact ramp rates, such as 2°C/min, rather than uncontrolled rapid heating.
This slow, controlled rise in temperature prevents thermal shock and ensures uniform reduction across the material.
Holding Times and Stability
Beyond the ramp rate, the furnace maintains specific temperatures for set durations.
This "holding time" ensures the reduction process is thermodynamically complete.
It allows the metal particles to stabilize on the support structure before cooling.
Engineering Surface Chemistry and Selectivity
Adjusting SMSI
The furnace temperature directly influences the Strong Metal-Support Interaction (SMSI).
SMSI describes how strongly the Pd-Ni particles bind electronically and physically to the ZrO2 support.
By adjusting the reduction temperature, you can optimize this interaction to prevent particle sintering (clumping) and enhance stability.
Creating Oxygen Vacancies
Heat treatment in this furnace dictates the formation of surface oxygen vacancies.
These vacancies are defects in the lattice structure that often act as active sites for adsorption.
Controlling the density of these vacancies is the key to optimizing the selectivity of the catalytic products.
Understanding the Trade-offs
The Risk of Sintering
While high temperatures are necessary for reduction, excessive heat is detrimental.
If the temperature overshoots or is held too long, the metal particles may aggregate (sinter).
This reduces the active surface area, significantly lowering the catalyst's overall efficiency.
Sensitivity to Atmosphere
The effectiveness of the furnace relies entirely on the purity and flow of the reducing gas (hydrogen).
Inconsistent gas flow or impurities can lead to incomplete reduction.
This results in a catalyst with mixed oxidation states, leading to unpredictable performance and poor selectivity.
Making the Right Choice for Your Goal
To maximize the potential of your Pd-Ni/ZrO2 catalysts, you must align your furnace programming with your specific catalytic objectives.
- If your primary focus is Product Selectivity: Prioritize precise temperature ceilings to fine-tune the oxygen vacancies and SMSI without altering the particle size.
- If your primary focus is Catalyst Stability: Utilize slower heating rates (e.g., 2°C/min) to ensure a gradual development of the metal-support interface, preventing future deactivation.
Ultimately, the programmable furnace allows you to transition from simply heating materials to engineering their atomic properties for specific chemical outcomes.
Summary Table:
| Feature | Impact on Catalyst | Key Benefit |
|---|---|---|
| Programmable Ramp Rate | Prevents thermal shock & sintering | Uniform particle size distribution |
| Controlled H2 Atmosphere | Efficient oxide-to-alloy reduction | High density of active metal sites |
| Precise Holding Times | Stabilizes Strong Metal-Support Interaction (SMSI) | Enhanced catalyst longevity and stability |
| High-Temp Accuracy | Tunes oxygen vacancy density | Optimized chemical selectivity and yield |
Elevate Your Material Engineering with KINTEK
Don't settle for unpredictable catalyst performance. KINTEK’s advanced laboratory furnaces are engineered specifically for precision-heavy applications like the activation of Pd-Ni/ZrO2 catalysts.
Backed by expert R&D and world-class manufacturing, we provide Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific research or production requirements. Whether you need exact 2°C/min ramp rates or specialized gas atmosphere controls, our high-temperature solutions ensure you can engineer atomic properties with confidence.
Ready to optimize your catalytic selectivity and stability?
Contact KINTEK today to discuss your custom furnace needs
Visual Guide
References
- Yuze Wu, He Tian. Preparation of a Pd/Ni Bimetallic Catalyst and its Application in the Selective Hydrogenation of Phenol. DOI: 10.61187/ita.v3i2.209
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- Why is a high-temperature tube furnace required for the post-treatment of composite anode materials in argon?
- What is the function of a laboratory high-temperature tube furnace? Driving Topotactic Reduction of Nickelate Films
- What types of production processes benefit from the thermal uniformity of tube furnaces? Enhance Precision in Material Processing
- How to operate a tubular furnace? A 5-Phase Guide for Safe and Repeatable Results
- Why is a high-precision tube furnace required during Fe-Mn catalyst synthesis? Control Morphology and CNF Quality
- What are the limitations of horizontal tube furnaces? Manage Space, Temperature, and Handling Challenges
- Why is a secondary high-temperature activation in a tubular furnace required? Unlock Peak Catalyst Performance
- How are rotary tube furnaces used in the mining and metallurgy industry? Boost Efficiency in Metal Processing



















