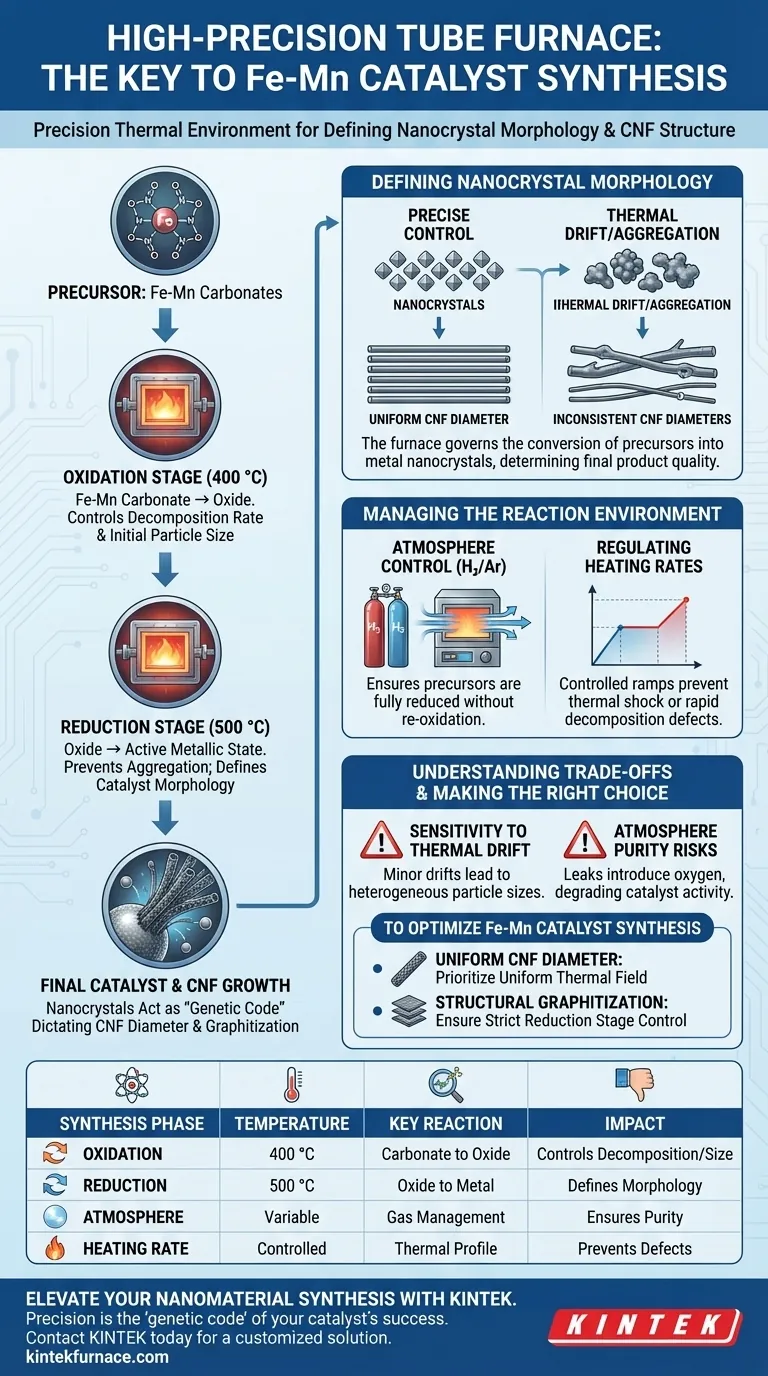
A high-precision tube furnace is required to maintain a strictly controlled thermal environment during the critical oxidation and reduction phases of Fe-Mn catalyst synthesis. It ensures the accurate transformation of Fe-Mn carbonates into oxides at 400 °C and their subsequent reduction to metal at 500 °C, which directly determines the size and uniform distribution of the resulting metal nanocrystals.
The core function of the furnace is not simply to heat the material, but to define the catalyst's morphology. The size and distribution of the metal particles formed during these thermal stages act as the "genetic code" that dictates the diameter and graphitization structure of the final Carbon Nanofibers (CNF).

The Link Between Temperature and Catalyst Morphology
Defining Nanocrystal Size
The primary role of the tube furnace is to govern the conversion of precursors into metal nanocrystals. By holding the oxidation stage precisely at 400 °C, the furnace controls the decomposition rate of Fe-Mn carbonates.
Controlling Particle Distribution
Following oxidation, the reduction stage at 500 °C transforms the oxides into active metallic states. Precise temperature control prevents the random aggregation of these particles, ensuring a uniform distribution rather than irregular clusters.
dictating Carbon Nanofiber (CNF) Structure
The morphology of the metal particles formed in the furnace is the determining factor for the final product. These nanoscale particles serve as the growth seeds for Carbon Nanofibers (CNF); their size strictly defines the fiber's growth diameter and structural quality (graphitization).
Managing the Reaction Environment
Atmosphere Control
The synthesis requires switching between distinct chemical environments—oxidative for the carbonate decomposition and reductive for the oxide transformation. A tube furnace excels at maintaining a stable atmosphere (such as Hydrogen/Argon mixtures) to ensure precursors are fully reduced without re-oxidizing.
Regulating Heating Rates
Beyond static temperature setpoints, the rate of heating and dwelling time are critical variables. Controlled heating ramps prevent thermal shock or rapid decomposition that could lead to structural defects in the catalyst support or the active metal sites.
Understanding the Trade-offs
Sensitivity to Thermal Drift
The formation of nanocrystals is thermodynamically sensitive. Even minor temperature drifts can alter the surface diffusion energy of atoms, leading to heterogeneous particle sizes that will result in inconsistent CNF diameters.
Atmosphere Purity Risks
While tube furnaces provide excellent atmosphere control, they rely on the integrity of the gas flow. Incomplete purging or leaks can introduce oxygen during the reduction phase, compromising the purity of the metal nanocrystals and degrading the catalyst's final activity.
Making the Right Choice for Your Goal
To optimize your Fe-Mn catalyst synthesis, align your equipment settings with your specific objectives:
- If your primary focus is Uniform CNF Diameter: Prioritize a furnace with exceptional thermal field uniformity to ensure every precursor particle experiences the exact same nucleation temperature.
- If your primary focus is Structural Graphitization: Ensure strict control over the reduction stage atmosphere and dwell time to maximize the crystallinity of the metal seed particles.
The precision of your thermal processing equipment is the single biggest variable in transitioning from a chemical precursor to a high-performance nanostructure.
Summary Table:
| Synthesis Phase | Temperature | Key Reaction | Impact on Final Product |
|---|---|---|---|
| Oxidation | 400 °C | Fe-Mn carbonate to oxide | Controls decomposition rate & initial particle size |
| Reduction | 500 °C | Oxide to active metallic state | Prevents aggregation; defines catalyst morphology |
| Atmosphere Control | Variable | Oxidative/Reductive gas management | Ensures purity and prevents re-oxidation of metal sites |
| Heating Rate | Controlled Ramps | Thermal profile management | Prevents structural defects and thermal shock |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is the 'genetic code' of your catalyst’s success. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, and Vacuum furnace systems designed to meet the rigorous demands of Fe-Mn catalyst development. Whether you need uniform thermal fields for consistent CNF diameters or advanced atmosphere control for CVD processes, our equipment is fully customizable for your unique lab requirements.
Ready to achieve superior material morphology? Contact KINTEK today for a customized solution.
Visual Guide
References
- Minki Sung, Seong‐Ho Yoon. Preparation Uniform Thin Tubular Carbon Nanofiber Using Novel Bimetallic Catalyst at Low Temperature and Its Structural Feature. DOI: 10.1021/acsomega.4c10295
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the primary function of a tube furnace in CVD COF synthesis? Achieve Precision 2D Film Growth
- What is the function of quartz tube vacuum sealing in Fe3GaTe2 crystal growth? Achieve High-Purity Results
- What conditions does a continuous flow fixed-bed quartz reactor provide? Master CO Oxidation Testing with Cobalt Oxide
- How does a laboratory tube sintering furnace facilitate the synthesis of BiCuSeO? Master Precise Thermal Diffusion
- What is the central design feature of a Quartz Tube Furnace? Unlock Real-Time Visual Monitoring in High-Temp Experiments
- Why is a precision tube furnace required for Nitrogen-doped SiOC synthesis? Ensure Perfect Structural Integrity
- How does a high-temperature tube furnace facilitate the pyrolysis stage in FeNC catalysts? Precision Thermal Processing
- What is the recommended procedure for atmosphere control in a vacuum tube furnace? Optimize Your High-Temperature Processes



















