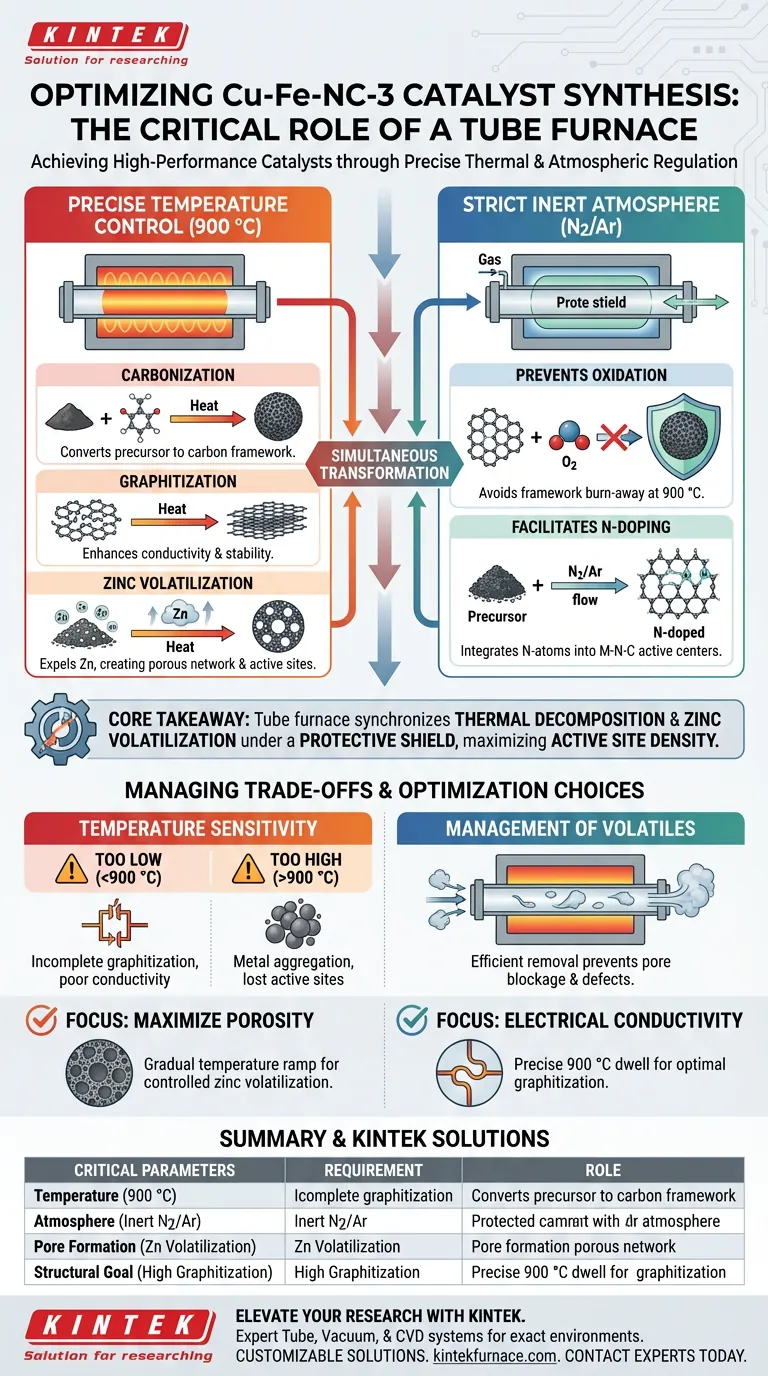
For the high-temperature pyrolysis of Cu-Fe-NC-3 catalysts, a tube furnace serves as a critical reaction chamber that provides two non-negotiable conditions: a precisely controlled temperature of 900 °C and a strict inert atmosphere.
This environment is essential to drive the simultaneous carbonization of the precursor and the volatilization of specific elements (specifically zinc), which transforms the raw materials into a highly graphitized Metal-Nitrogen-Carbon framework with abundant active sites.
Core Takeaway: The tube furnace is not merely a heat source; it is a physicochemical regulator. Its primary function for Cu-Fe-NC-3 synthesis is to synchronize the thermal decomposition of precursors with the volatilization of pore-forming agents (zinc) under a protective shield, preventing oxidation while maximizing active site density.

The Role of Thermal Precision
Establishing the 900 °C Environment
The synthesis of Cu-Fe-NC-3 relies on reaching and maintaining a specific thermal plateau, typically at 900 °C.
This temperature is not arbitrary; it provides the activation energy required to convert the organic precursor into a stable inorganic framework.
Driving Carbonization and Graphitization
At this high temperature, the furnace drives the carbonization of the precursor material.
This process rearranges the carbon atoms, resulting in a highly graphitized structure. High graphitization is crucial for the material's electrical conductivity and chemical stability in electrochemical applications.
Creating Porosity via Zinc Volatilizations
A unique function of the thermal environment for this specific catalyst is the controlled volatilization of zinc.
As the furnace heats the precursor, zinc elements are vaporized and expelled from the material. This removal creates a network of pores, significantly increasing the specific surface area and exposing more active sites.
The Importance of Atmospheric Control
Strict Inert Atmosphere Protection
The tube furnace must maintain a strict inert atmosphere (typically nitrogen or argon) throughout the process.
This "protective blanket" is critical because, at 900 °C, the carbon framework would instantly burn away (oxidize) if exposed to oxygen.
Facilitating Nitrogen Doping
Within this protected environment, nitrogen-doping reactions occur simultaneously with carbonization.
The inert atmosphere ensures that nitrogen atoms—derived from the precursor or the gas flow—are successfully integrated into the carbon lattice to form the Metal-Nitrogen-Carbon (M-N-C) active centers, rather than reacting with atmospheric oxygen.
Understanding the Trade-offs
Temperature Sensitivity
While 900 °C is the target for Cu-Fe-NC-3, deviating from this precision introduces significant risks.
If the temperature is too low, the graphitization will be incomplete, leading to poor conductivity. If too high, metal atoms may aggregate into large particles rather than forming the desired dispersed active sites.
Management of Volatiles
The volatilization of zinc is necessary for pore creation, but it presents a process challenge.
The furnace's gas flow system must be efficient enough to expel these volatile decomposition products from the reaction zone. Failure to remove these byproducts can lead to distinct defects or pore blockage in the final catalyst structure.
Making the Right Choice for Your Goal
To maximize the performance of your Cu-Fe-NC-3 catalyst, tailor your focus based on your specific structural requirements:
- If your primary focus is maximizing porosity: Ensure the furnace maintains a steady temperature ramp that allows zinc volatilization to occur gradually, preventing structural collapse before the carbon framework solidifies.
- If your primary focus is electrical conductivity: Prioritize the precision of the 900 °C dwell time, as this directly dictates the degree of graphitization and the stability of the final carbon matrix.
Success depends on viewing the tube furnace not as a passive heater, but as a dynamic tool for sculpting the atomic architecture of your catalyst.
Summary Table:
| Critical Parameter | Requirement | Role in Cu-Fe-NC-3 Synthesis |
|---|---|---|
| Temperature | 900 °C | Drives carbonization, graphitization, and zinc volatilization. |
| Atmosphere | Strict Inert (N2/Ar) | Prevents oxidation and facilitates essential nitrogen-doping. |
| Pore Formation | Zinc Volatilization | Creates high surface area and exposes catalytic active sites. |
| Structural Goal | High Graphitization | Ensures electrical conductivity and chemical stability. |
Elevate Your Catalyst Research with KINTEK
Precise thermal regulation and atmospheric purity are the difference between a failed precursor and a high-performance Cu-Fe-NC-3 catalyst. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Tube, Vacuum, and CVD systems designed to maintain the exact 900 °C plateaus and strict inert environments your synthesis requires.
Whether you need a standard setup or a customizable high-temp furnace for unique laboratory needs, our equipment ensures maximum active site density and superior graphitization for your materials.
Ready to optimize your pyrolysis process? Contact our technical experts today to find the perfect furnace for your lab.
Visual Guide
References
- Kun Liu, Xin Chen. Highly efficient Fe–Cu dual-site nanoparticles supported on black pearls 2000 (carbon black) as oxygen reduction reaction catalysts for Al–air batteries. DOI: 10.1039/d3ra07925b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why is a tube reduction furnace used for the pre-reduction of CeAlOx/NiO/Ni-foam catalysts? Essential Catalyst Prep
- What role does a tube furnace play in g-C3N4 thin film preparation? Optimize Your Hot-Wall CVD Synthesis
- What changes occur in materials processed in a tube furnace? Discover Physical, Chemical, and Heat Treatment Transformations
- What safety precautions should be followed when operating a multi zone tube furnace? Ensure Safe and Efficient Lab Operations
- How does a high-temperature tube furnace combustion system function in food waste analysis? Master Ultimate Analysis
- What are some common applications of a High Temperature Tube Furnace? Unlock Precision in Materials Science
- What role does the hot zone of a horizontal tube furnace play in CVD for ITO? Master Thin Film Precision
- How does the melt-diffusion process for Te1S7 use tube furnaces? Achieve High-Precision Molecular Confinement



















