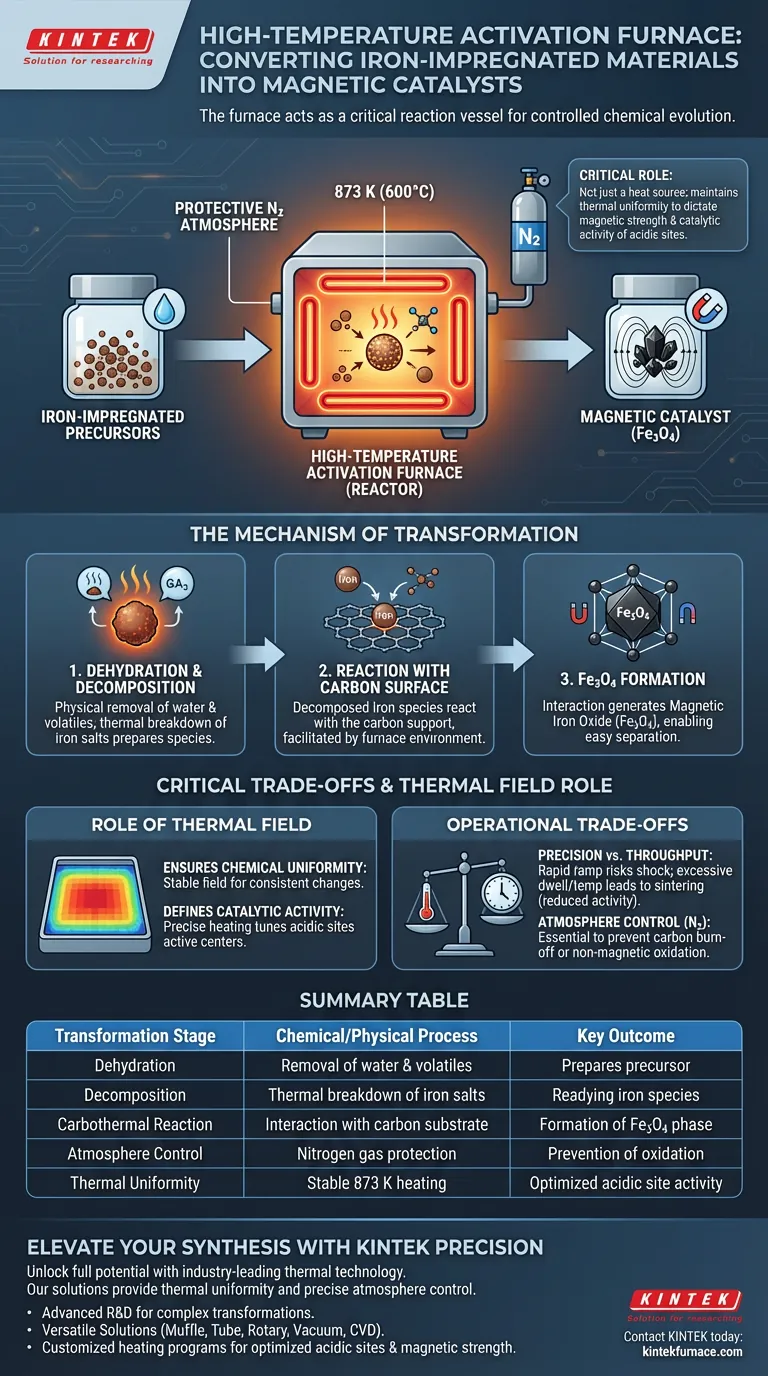
The high-temperature activation furnace serves as the critical reaction vessel for converting iron-impregnated precursors into functional magnetic catalysts. Under a protective nitrogen atmosphere, typically at 873 K (600°C), it drives a reductive transformation that dehydrates and decomposes iron salts, ultimately reacting them with the carbon substrate to form magnetic iron oxide (Fe3O4).
The furnace is not merely a heat source; it acts as a reactor for controlled chemical evolution. Its ability to maintain thermal uniformity directly dictates the resulting magnetic strength and the catalytic activity of the material's acidic sites.

The Mechanism of Transformation
Dehydration and Decomposition
The process begins with the physical removal of water and volatile components. As the furnace ramps up to the target temperature, the iron salt precursors undergo dehydration.
Following dehydration, the salts decompose thermally. This prepares the iron species for the final chemical bonding required to activate the material.
Reaction with the Carbon Surface
The defining chemical event occurs when the decomposed iron species interact with the carbon support. The furnace environment facilitates a reaction between these precursors and the carbon surface.
This interaction generates magnetic iron oxide (Fe3O4). This specific oxide phase is responsible for the material’s magnetic properties, allowing for easy separation of the catalyst after use.
The Role of the Thermal Field
Ensuring Chemical Uniformity
The furnace provides a stable thermal field, which is essential for consistent chemical changes throughout the material batch.
If the heat distribution is uneven, the conversion of iron salts will be incomplete or inconsistent. This leads to a catalyst with unpredictable performance characteristics.
Defining Catalytic Activity
The specific heating program (e.g., 873 K for 1 hour) does more than create magnetism; it tunes the chemical behavior of the surface.
The uniformity of this temperature directly determines the final activity of the acidic sites. These sites are the active centers where future catalytic reactions will actually take place.
Critical Trade-offs in Operation
Temperature Precision vs. Throughput
Achieving the precise crystalline structure of Fe3O4 requires strict adherence to the temperature profile. Ramping the temperature too quickly to save time can lead to thermal shock or incomplete decomposition.
Conversely, excessive dwell times or temperatures exceeding the optimal 873 K range may lead to sintering. Sintering reduces the surface area and diminishes the activity of the acidic sites.
Atmosphere Control
The primary reference highlights the necessity of nitrogen protection. This inert atmosphere prevents uncontrolled oxidation.
Without this protection, the carbon support could consume itself (burn off) or the iron could oxidize into non-magnetic phases (like Fe2O3), rendering the catalyst useless for its intended magnetic separation applications.
Making the Right Choice for Your Goal
To maximize the efficacy of your catalyst synthesis, align your furnace operation with your specific performance metrics.
- If your primary focus is Magnetic Separation: Prioritize temperature uniformity and strict atmosphere control to ensure the maximum yield of the Fe3O4 phase.
- If your primary focus is Chemical Reactivity: Focus on the precise heating duration and ramp rates to optimize the distribution and strength of the acidic active sites.
Control the thermal field, and you control the chemistry.
Summary Table:
| Transformation Stage | Chemical/Physical Process | Key Outcome |
|---|---|---|
| Dehydration | Removal of water & volatiles | Prepares precursor for decomposition |
| Decomposition | Thermal breakdown of iron salts | Readying iron species for bonding |
| Carbothermal Reaction | Interaction with carbon substrate | Formation of Magnetic Fe3O4 phase |
| Atmosphere Control | Nitrogen gas protection | Prevention of carbon burn-off/oxidation |
| Thermal Uniformity | Stable 873 K heating | Optimized acidic site catalytic activity |
Elevate Your Catalyst Synthesis with KINTEK Precision
Unlock the full potential of your material transformations with KINTEK’s industry-leading thermal technology. Whether you are developing magnetic catalysts or advanced carbon substrates, our high-temperature solutions provide the thermal uniformity and precise atmosphere control essential for consistent chemical evolution.
Why Choose KINTEK?
- Advanced R&D: Systems designed for complex reductive transformations and dehydration processes.
- Versatile Solutions: Choose from our Muffle, Tube, Rotary, Vacuum, and CVD systems to match your specific production scale.
- Customized for Excellence: We tailor heating programs to optimize your material’s acidic sites and magnetic strength.
Don't let uneven heat distribution compromise your research. Contact KINTEK today to discuss your unique needs and see how our expert-engineered furnaces can refine your chemical outcomes.
Visual Guide
References
- Luigi di Bitonto, Carlo Pastore. A Closed-Loop Biorefinery Approach for the Valorization of Winery Waste: The Production of Iron-Sulfonated Magnetic Biochar Catalysts and 5-Hydroxymethyl Furfural from Grape Pomace and Stalks. DOI: 10.3390/catal14030185
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a high-purity argon protective environment necessary during the mechanical alloying of Cu-Al2O3 powder?
- What is the purpose of the secondary heat treatment in an annealing furnace? Enhance S@Se-ZnS/HSC Material Stability
- Why is the use of a programmable box furnace critical for the preparation of U0.92Mn3Si2C? Ensure Synthesis Precision
- Why is argon gas used for 800HT alloy experiments? Protect Material Integrity with Inert Atmosphere Control
- What is a retort furnace and what are its key features? Discover Precision Heating for Superior Material Processing
- What is the role of a laboratory high-temperature annealing furnace in preparing amorphous (InxGa1-x)2O3 thin films?
- Why is a laboratory high-temperature annealing furnace necessary after the initial formation of a perovskite thin film?
- What are the risk mitigation strategies for atmosphere furnace operation? Ensure Safe and Efficient Lab Processes



















