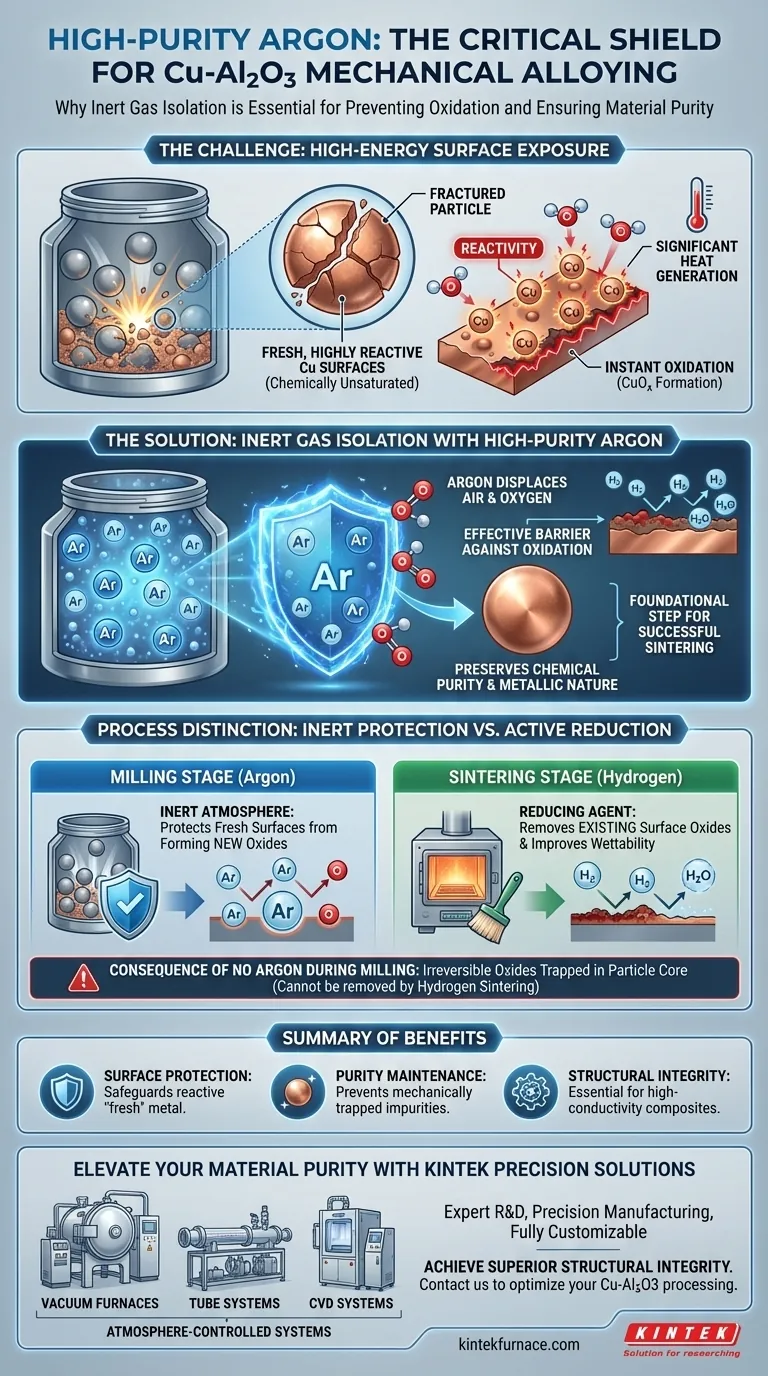
A high-purity argon environment serves as a critical barrier against oxidation. During the mechanical alloying of Cu-Al2O3, high-energy grinding continuously fractures particles, exposing fresh, highly reactive copper surfaces. Argon displaces the air in the milling jar, preventing oxygen from contacting these surfaces and compromising the material's purity.
Mechanical alloying generates significant heat and creates unstable, reactive surface areas that make copper highly susceptible to contamination. An argon atmosphere preserves the chemical purity of the powder, which is a strictly necessary prerequisite for achieving high-quality results in the subsequent sintering phase.

The Physics of Fresh Surfaces
High-Energy Surface Exposure
The mechanical alloying process involves intense collisions between grinding balls and the powder mixture.
This action repeatedly fractures the material, exposing fresh metal surfaces that have never been exposed to the atmosphere before.
Heightened Reactivity
These newly exposed surfaces differ significantly from the exterior of a resting particle.
Because the surface atoms are chemically "unsaturated," they are highly reactive and seek to stabilize by bonding with surrounding elements.
Without protection, these surfaces would instantly react with oxygen in the air, forming unwanted copper oxide layers.
The Role of Inert Gas Isolation
Preventing Copper Oxidation
The primary function of high-purity argon is isolation.
By filling the ball milling jar with argon, you create an inert environment where oxygen is effectively absent.
This allows the prolonged grinding process to continue without the copper powder suffering from oxidation, preserving the metallic nature of the matrix.
Ensuring Chemical Purity
The integrity of a Cu-Al2O3 composite depends on the purity of its constituents.
If oxidation occurs during milling, impurities are mechanically trapped within the composite structure.
Using argon ensures the final powder maintains the chemical purity required for its intended electrical and mechanical applications.
Understanding Process Distinctions
Inert Protection vs. Active Reduction
It is critical to distinguish between the protection required during milling and the atmosphere used during sintering.
Argon is used during milling because it is inert; it protects fresh surfaces from forming new oxides.
Hydrogen, typically used in the later sintering stage, acts as a reducing agent to remove existing surface oxides and improve wettability.
The Consequence of Milling Without Argon
Failing to use argon during the milling phase creates irreversible damage.
While hydrogen sintering can clean surface oxides later, it cannot easily remove oxides that have been mechanically alloyed into the particle core during grinding.
Therefore, argon protection during milling is the foundational step that determines the success of the sintering phase.
Making the Right Choice for Your Goal
To maximize the performance of your Cu-Al2O3 composite, you must match the atmosphere to the specific stage of processing.
- If your primary focus is preventing contamination during milling: Ensure the milling jar is sealed with high-purity argon to isolate reactive fresh surfaces from oxygen.
- If your primary focus is maximizing interfacial bonding: Rely on argon during milling to preserve purity, ensuring the material is ready for the high-performance sintering that follows.
Strict atmosphere control during mechanical alloying is the only way to guarantee the structural integrity required for high-conductivity composite materials.
Summary Table:
| Feature | Purpose in Cu-Al2O3 Mechanical Alloying |
|---|---|
| Inert Atmosphere | Displaces oxygen to prevent immediate surface oxidation |
| Surface Protection | Safeguards highly reactive "fresh" metal exposed during grinding |
| Purity Maintenance | Prevents oxides from being mechanically trapped in the composite core |
| Argon vs. Hydrogen | Argon isolates during milling; Hydrogen reduces oxides during sintering |
Elevate Your Material Purity with KINTEK Precision Solutions
Don't let oxidation compromise your high-performance composites. KINTEK provides industry-leading high-temperature lab furnaces and atmosphere-controlled systems—including Vacuum, Tube, and CVD systems—specifically designed to maintain the strict environments required for mechanical alloying and sintering. Backed by expert R&D and precision manufacturing, our equipment is fully customizable to meet your unique metallurgical needs.
Ready to achieve superior structural integrity? Contact KINTEK today to discuss how our advanced furnace technology can optimize your Cu-Al2O3 powder processing.
Visual Guide
References
- Tawfik M. Ahmed. Development and characterization of Cu-Al2O3 metal matrix composites through powder metallurgy techniques. DOI: 10.33545/26646536.2025.v7.i2a.137
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the different configurations available for inert atmosphere furnaces? Find Your Perfect Match for Heat Treatment
- Why is the temperature control of a high-precision resistance furnace essential for B4C/Al composites? Gain Control
- What types of furnaces are specially designed for processing in inert atmospheres? Explore Sealed Systems for Oxidation-Free Results
- What are the advantages of controlled atmosphere furnaces over the older types? Boost Efficiency, Quality, and Safety
- Why is a laboratory high-temperature furnace required for ML-MFC cathodes? Ensure Stable Pre-oxidation
- Why is an Argon-Hydrogen gas mixture used in aerodynamic levitation? Achieve Pure Metal Melting and Precision Control
- How does an inert atmosphere furnace work? Master Controlled Heating for Oxidation-Free Results
- What are the primary purposes of a controlled atmosphere furnace? Achieve Precise Material Processing and Protection



















