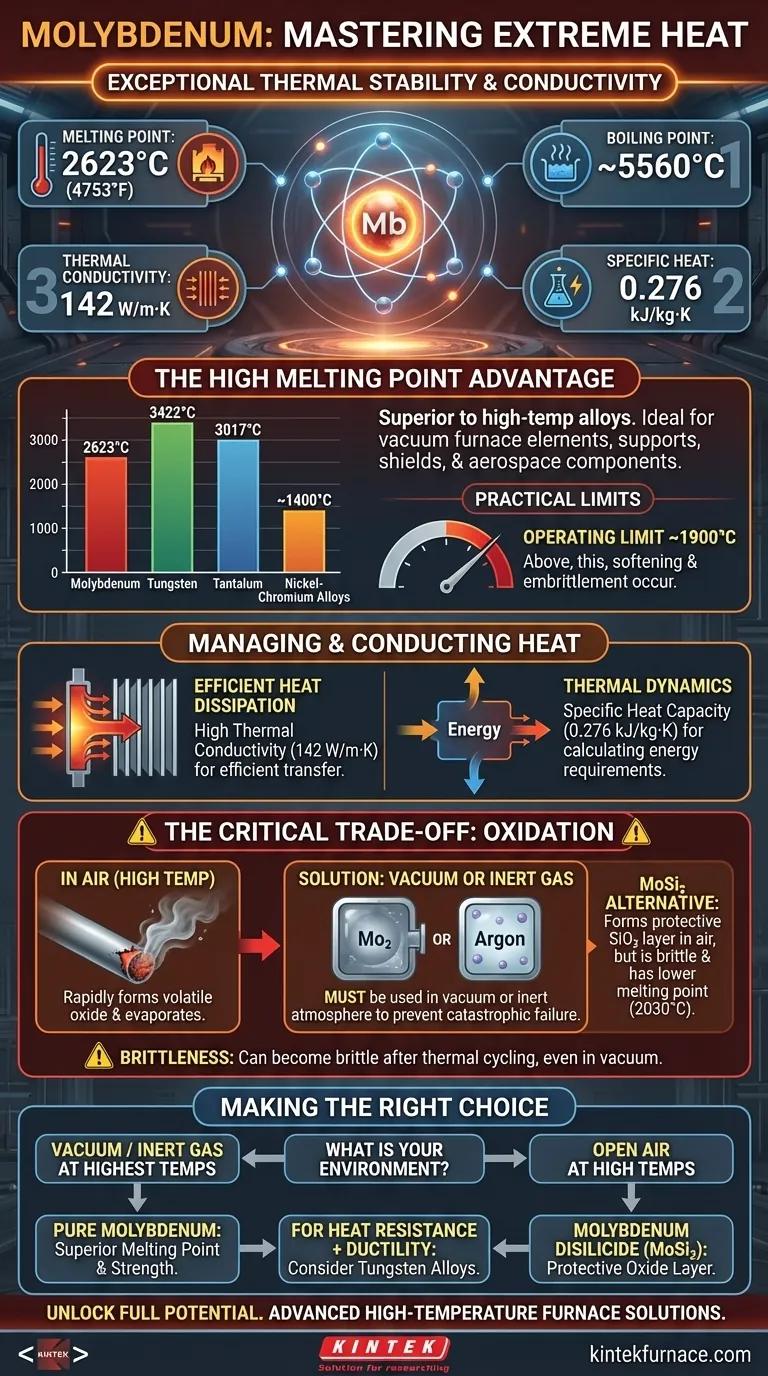
At its core, molybdenum is a refractory metal defined by its exceptional thermal stability and conductivity. Its key thermal properties include a melting point of 2623°C, a boiling point near 5560°C, a thermal conductivity of 142 W/m·K, and a specific heat of 0.276 kJ/kg·K at room temperature. These figures place it in an elite class of materials capable of withstanding extreme heat.
Molybdenum's value stems from its extremely high melting point and good thermal conductivity. However, these benefits come with a critical trade-off: its poor oxidation resistance requires it to be used in a vacuum or inert atmosphere at high temperatures.

The Significance of a High Melting Point
Molybdenum's most notable characteristic is its ability to maintain integrity at temperatures that would melt most common metals. This places it firmly in the category of refractory metals.
A Refractory Metal Benchmark
The melting point of molybdenum, 2623°C (4753°F), is one of the highest among the elements. It is surpassed by only a few others, such as tungsten and tantalum. This makes it far superior to high-temperature nickel-chromium alloys for extreme heat applications.
Applications in Extreme Heat
This high melting point makes molybdenum a primary material for components inside vacuum furnaces, such as heating elements, supports, and shields. It is also used in glass manufacturing electrodes and aerospace applications where components face immense thermal stress.
Practical Temperature Limits
Despite its high melting point, the practical operating temperature for pure molybdenum is typically limited to around 1900°C. Above this temperature, it begins to soften and can become excessively brittle, compromising its structural integrity.
How Molybdenum Manages and Conducts Heat
Beyond simply resisting melting, molybdenum's other thermal properties dictate how it behaves as a functional component in a thermal system.
High Thermal Conductivity
With a thermal conductivity of 142 W/m·K, molybdenum is very effective at transferring heat. This is a valuable property for applications like heat sinks or electrical contacts, where efficiently dissipating heat is as important as withstanding it.
Specific Heat Capacity
Molybdenum's specific heat of 0.276 kJ/kg·K is a measure of the energy required to raise its temperature. While not unusually high or low, this value is a critical parameter for calculating thermal dynamics and energy consumption in systems using molybdenum components.
Understanding the Trade-offs: Oxidation and Brittleness
No material is perfect, and molybdenum's primary weakness is its reaction to oxygen at elevated temperatures. This is the single most important factor to consider when designing with it.
The Critical Need for a Vacuum
When heated in the presence of oxygen, molybdenum rapidly forms a volatile oxide that sublimates, causing the material to literally evaporate. To prevent this catastrophic failure, it must be used in a vacuum or an inert gas atmosphere (like argon or nitrogen) at high temperatures.
A Note on Molybdenum Disilicide (MoSi₂)
To address the oxidation problem, engineers developed compounds like molybdenum disilicide (MoSi₂). This material forms a protective, self-healing layer of silicon dioxide (SiO₂) when heated in air, allowing it to function at high temperatures without a vacuum. However, MoSi₂ has a lower melting point (2030°C) and is very brittle at room temperature.
Brittleness After Thermal Cycling
Even in a vacuum, molybdenum can become brittle after being held at very high temperatures. This reduces its resistance to mechanical shock and must be factored into the design and handling of any components.
Making the Right Choice for Your Application
Selecting the right material requires balancing ideal properties with practical limitations. Your final decision depends entirely on the operational environment.
- If your primary focus is the highest possible temperature in a vacuum: Pure molybdenum is an excellent choice due to its superior melting point and strength at extreme heat.
- If your application operates at high temperatures in open air: You must use an alternative like molybdenum disilicide (MoSi₂) to benefit from its protective oxide layer.
- If your design demands both heat resistance and ductility: Carefully evaluate the potential for molybdenum to become brittle and consider tungsten alloys or other refractory materials as alternatives.
Ultimately, understanding the trade-off between molybdenum's exceptional heat resistance and its environmental sensitivity is the key to using it successfully.
Summary Table:
| Property | Value | Significance |
|---|---|---|
| Melting Point | 2623°C (4753°F) | Exceptional high-temperature stability |
| Boiling Point | ~5560°C | Extreme heat resistance |
| Thermal Conductivity | 142 W/m·K | Efficient heat dissipation |
| Specific Heat Capacity | 0.276 kJ/kg·K | Key for thermal dynamics calculations |
| Practical Operating Limit | ~1900°C | Maximum recommended use temperature |
Unlock the full potential of molybdenum in your high-temperature processes.
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements.
Contact our experts today to discuss how our high-temperature furnace solutions can optimize your application's performance and reliability.
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- What is the role of vacuum pumps in a vacuum heat treatment furnace? Unlock Superior Metallurgy with Controlled Environments
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- Why is a high vacuum essential for Ti-6Al-4V sintering? Protect Your Alloys from Embrittlement
- What are the benefits of using a high-temperature vacuum furnace for the annealing of ZnSeO3 nanocrystals?



















