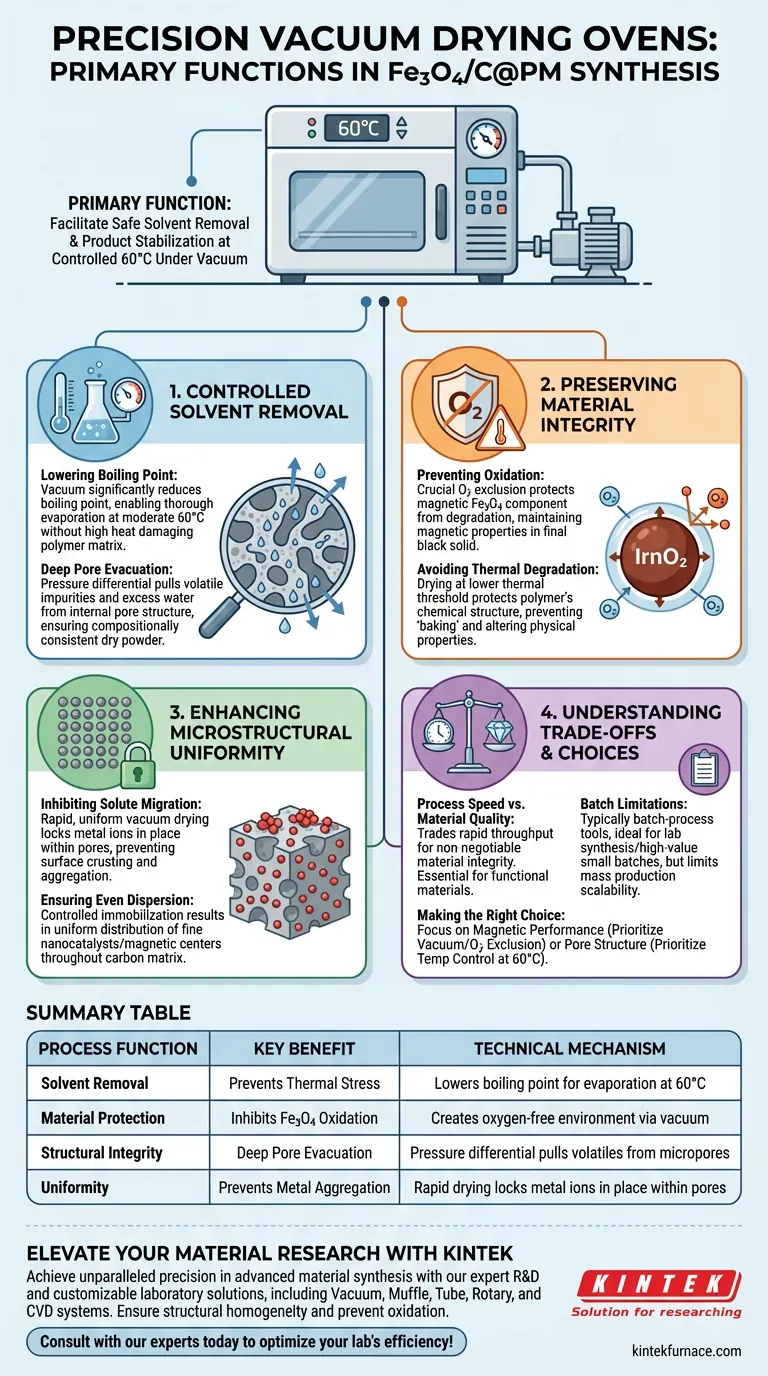
The primary function of a precision vacuum drying oven in this synthesis is to facilitate the safe removal of solvents and the stabilization of the final product. By operating under vacuum conditions at a controlled 60°C, the oven eliminates residual moisture and low-boiling organic solvents without subjecting the delicate magnetic carbon porous polymers (Fe3O4/C@PM) to damaging thermal stress or oxidative environments.
The vacuum environment fundamentally alters the drying kinetics, allowing for the complete evacuation of deep-pore solvents at reduced temperatures. This process is critical for preventing the oxidation of magnetic iron components and ensuring the structural homogeneity of the final black solid product.
The Mechanics of Controlled Solvent Removal
Lowering the Boiling Point
The synthesis of Fe3O4/C@PM involves porous structures trapped with moisture and organic solvents.
By creating a vacuum, the oven significantly reduces the boiling point of these liquids. This allows for thorough evaporation at a moderate 60°C, ensuring the material is dried completely without requiring high heat that could damage the polymer matrix.
Deep Pore Evacuation
Standard atmospheric drying often fails to remove solvents trapped deep within micropores.
The pressure differential created by the vacuum pulls volatile impurities and excess water out from the internal pore structure. This ensures the production of a compositionally consistent dry powder rather than a material with trapped liquid pockets.
Preserving Material Integrity
Preventing Oxidation of Magnetic Components
The most critical role of the vacuum is the exclusion of oxygen.
The magnetic component (Fe3O4) is susceptible to oxidation at high temperatures, which would degrade its magnetic properties. Vacuum drying removes oxygen from the chamber, ensuring the final product remains a stable, magnetic black solid.
Avoiding Thermal Degradation
High temperatures can cause premature differentiation or structural collapse of the precursors.
By enabling drying at a lower thermal threshold, the process protects the chemical structure of the polymer. This prevents the "baking" effect that alters the physical properties of the final material.
Enhancing Microstructural Uniformity
Inhibiting Solute Migration
During standard drying, solvents moving to the surface often carry dissolved metal ions with them, leading to crusting.
Vacuum drying is rapid and uniform, which effectively locks the metal ions in place within the pores. This prevents the metal aggregation that typically occurs during solvent migration.
Ensuring Even Dispersion
The result of this controlled immobilization is a uniform distribution of components.
This facilitates the formation of fine, evenly dispersed nanocatalysts (or magnetic centers) throughout the carbon matrix, rather than having them clumped on the exterior surface.
Understanding the Trade-offs
Process Speed vs. Material Quality
Vacuum drying is generally an "extended period" process compared to high-heat atmospheric drying.
You trade rapid throughput for material integrity. If speed is the only metric, this method is inefficient; however, for functional materials like magnetic polymers, this time investment is non-negotiable to maintain performance.
Batch Limitations
Precision vacuum ovens are typically batch-process tools.
This limits scalability compared to continuous drying methods. While perfect for laboratory synthesis or high-value small batches, it can become a bottleneck in mass production scenarios.
Making the Right Choice for Your Goal
To maximize the quality of your Fe3O4/C@PM synthesis, consider your specific processing priorities:
- If your primary focus is Magnetic Performance: Prioritize the vacuum level over temperature; excluding oxygen is the single most important factor to prevent Fe3O4 oxidation.
- If your primary focus is Pore Structure: Ensure the temperature remains strictly controlled (e.g., 60°C) to prevent thermal collapse of the polymer pores while the solvent evacuates.
Ultimately, the vacuum oven is not just a drying tool, but a stabilization environment that defines the final chemical and physical quality of your magnetic polymer.
Summary Table:
| Process Function | Key Benefit | Technical Mechanism |
|---|---|---|
| Solvent Removal | Prevents Thermal Stress | Lowers boiling point for evaporation at 60°C |
| Material Protection | Inhibits Fe3O4 Oxidation | Creates an oxygen-free environment via vacuum |
| Structural Integrity | Deep Pore Evacuation | Pressure differential pulls volatiles from micropores |
| Uniformity | Prevents Metal Aggregation | Rapid drying locks metal ions in place within pores |
Elevate Your Material Research with KINTEK
Achieve unparalleled precision in your advanced material synthesis. Backed by expert R&D and manufacturing, KINTEK offers a wide range of laboratory solutions including Vacuum, Muffle, Tube, Rotary, and CVD systems. Our high-temperature furnaces are fully customizable to meet the unique needs of delicate processes like magnetic polymer stabilization, ensuring structural homogeneity and preventing oxidation.
Consult with our experts today to optimize your lab's efficiency!
Visual Guide
References
- Magnetic Carbon Porous Polymer Prepared from a New Suspended Emulsion for the Absorption of Heavy Metal Ions. DOI: 10.3390/polym17030257
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- What is the Bell Jar Furnace designed for? Achieve Ultra-Clean Processing for Sensitive Components
- What are the key features of a high-quality vacuum heat treatment furnace? Ensure Superior Heat Treatment Precision
- What advantages does a vacuum drying oven offer over a standard oven for Fe3Al and CNTs? Protect Your Composites
- How is forced cooling achieved in hot wall vacuum furnaces? Optimize Metallurgical Properties with Precision Cooling
- Why is a Vacuum Drying Oven necessary for KF-NaF-AlF3 electrolytes? Prevent Hydrolysis and Corrosion
- What automation features are present in modern vacuum furnaces? Boost Precision and Efficiency in Your Lab
- What are the key characteristics of vacuum furnaces? Achieve Superior Material Processing
- How do graphite heating elements function in vacuum furnaces? Unlocking Extreme Heat for Critical Processes



















