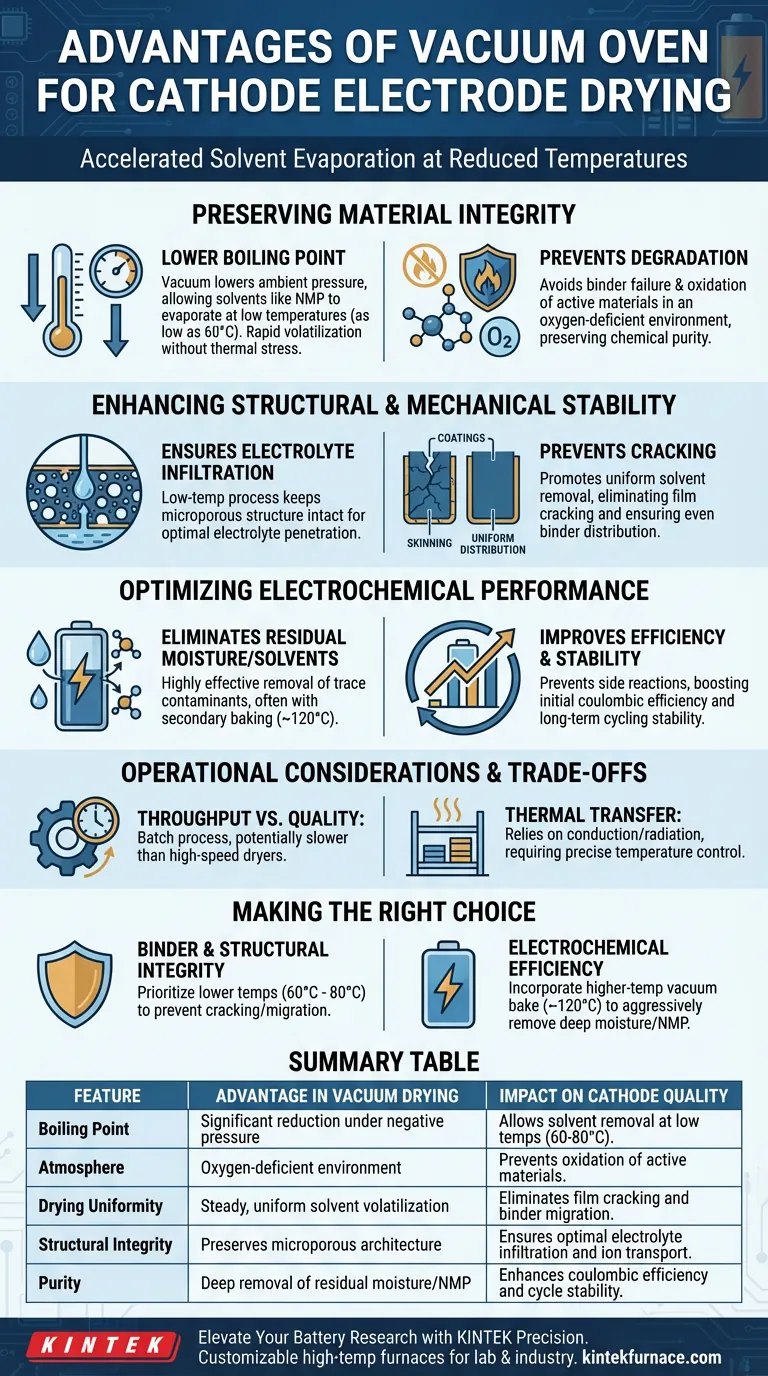
The primary advantage of using a vacuum oven for cathode electrode drying is the ability to accelerate solvent evaporation at significantly reduced temperatures. By lowering the ambient pressure, you can effectively remove solvents like N-Methyl-2-pyrrolidone (NMP) at temperatures as low as 60°C, ensuring the removal of volatiles without the thermal risks associated with standard atmospheric drying.
Vacuum drying decouples temperature from evaporation rates, allowing for the thorough removal of solvents and moisture without subjecting delicate cathode materials to degradative heat. This preserves the binder's integrity and the electrode's microporous structure, directly translating to superior electrochemical performance.
Preserving Material Integrity Through Low-Temperature Drying
The central challenge in cathode preparation is removing solvents without damaging the chemical structure of the electrode components.
Lowering the Boiling Point
Under standard atmospheric pressure, removing solvents like NMP requires high temperatures. A vacuum environment significantly lowers the boiling point of these solvents.
This allows for rapid volatilization at much lower temperatures (e.g., 60°C to 80°C), protecting the electrode from the harsh thermal stress required in conventional ovens.
Preventing Component degradation
High temperatures can cause the failure of binders used to hold the active materials together. When binders degrade, the mechanical strength of the electrode fails.
Additionally, excessive heat promotes the oxidation of active materials. Vacuum drying creates an oxygen-deficient environment that mitigates this risk, preserving the chemical purity of the cathode.
Enhancing Structural and Mechanical Stability
Beyond chemical preservation, vacuum drying is critical for maintaining the physical architecture of the electrode coating.
Ensuring Electrolyte Infiltration
For a battery to function efficiently, the electrolyte must be able to penetrate the cathode layer.
The low-temperature vacuum process ensures that the microporous structure of the cathode coating remains intact. This open structure is vital for facilitating optimal electrolyte infiltration and ion transport.
Preventing Cracking and Uneven Distribution
Rapid drying at high temperatures often leads to "skinning," where the surface dries faster than the interior, leading to film cracking or delamination.
Vacuum drying promotes a more uniform removal of solvents. This prevents cracking and ensures a uniform distribution of the binder between the active material and the current collector, enhancing the mechanical stability of the electrode.
Optimizing Electrochemical Performance
The ultimate goal of the drying process is to ensure the finished battery performs reliably over time.
Eliminating Residual Moisture and Solvents
Even trace amounts of NMP or moisture can be catastrophic for battery performance.
Vacuum ovens are highly effective at removing residual moisture adsorbed on electrode sheets, even from deep within porous agglomerates. This is often performed in a secondary baking stage (around 120°C).
Improving Efficiency and Stability
By removing these contaminants, vacuum drying prevents side reactions during the battery's charging and discharging cycles.
The result is a direct improvement in initial coulombic efficiency and long-term cycling stability, as the conductive network remains uncompromised by non-conductive oxide layers or decomposition products.
Operational Considerations and Trade-offs
While vacuum drying offers superior quality, it requires careful process management.
Throughput vs. Quality
Vacuum drying is inherently a batch or semi-continuous process that may be slower than high-speed hot air flotation dryers used in mass production.
Thermal Transfer Limitations
In a vacuum, heat transfer via convection is eliminated; heat must be transferred via conduction or radiation. This requires precise control of shelf temperatures to ensure the electrode sheets are heated uniformly without hot spots.
Making the Right Choice for Your Goal
The specific parameters of your vacuum drying process should be tuned to your most critical performance metrics.
- If your primary focus is Binder and Structural Integrity: Prioritize lower temperatures (approx. 60°C - 80°C) to prevent cracking and binder migration while relying on vacuum pressure to drive evaporation.
- If your primary focus is Electrochemical Efficiency: Incorporate a higher-temperature vacuum bake (approx. 120°C) as a final step to aggressively remove trace moisture and residual NMP deep within the pores.
By utilizing negative pressure to manipulate the evaporation threshold, you transform drying from a thermal brute-force operation into a precise, preservation-focused process.
Summary Table:
| Feature | Advantage in Vacuum Drying | Impact on Cathode Quality |
|---|---|---|
| Boiling Point | Significant reduction under negative pressure | Allows solvent removal at low temperatures (60-80°C) |
| Atmosphere | Oxygen-deficient environment | Prevents oxidation of active materials |
| Drying Uniformity | Steady, uniform solvent volatilization | Eliminates film cracking and binder migration |
| Structural Integrity | Preserves microporous architecture | Ensures optimal electrolyte infiltration and ion transport |
| Purity | Deep removal of residual moisture/NMP | Enhances coulombic efficiency and cycle stability |
Elevate Your Battery Research with KINTEK Precision
Achieve unmatched electrochemical stability and structural integrity for your electrode materials. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, Muffle, Tube, and CVD systems tailored for the most demanding laboratory and industrial applications. Whether you need to eliminate residual moisture or prevent thermal degradation, our customizable high-temp furnaces provide the precise control your unique projects require.
Ready to optimize your drying process? Contact our technical experts today to find the perfect solution for your lab.
Visual Guide
References
- Ka Chun Li, Xijun Hu. Single-step synthesis of titanium nitride-oxide composite and AI-driven aging forecast for lithium–sulfur batteries. DOI: 10.1039/d4ta00234b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- How is cooling achieved in vacuum furnaces? Master Controlled Cooling for Superior Material Properties
- What are the main functions of a vacuum carburizing furnace? Achieve Superior Heat Treatment with Precision
- What is a crucible furnace and what are its main components? Discover Its Key Parts and Uses
- What is the heat treatment in a vacuum oven? Achieve Superior Surface & Material Integrity
- What are the disadvantages of vacuum brazing? Understanding the trade-offs for your application
- Why is a vacuum drying oven preferred for ZIF-8 crystal precursors? Protect Porous Structures with Vacuum Drying
- How do customized vacuum furnaces contribute to energy efficiency? Unlock Cost Savings and Eco-Friendly Processing
- How does the mechanical drive system of a Floating-Zone furnace impact crystal quality? Ensuring Homogeneity



















