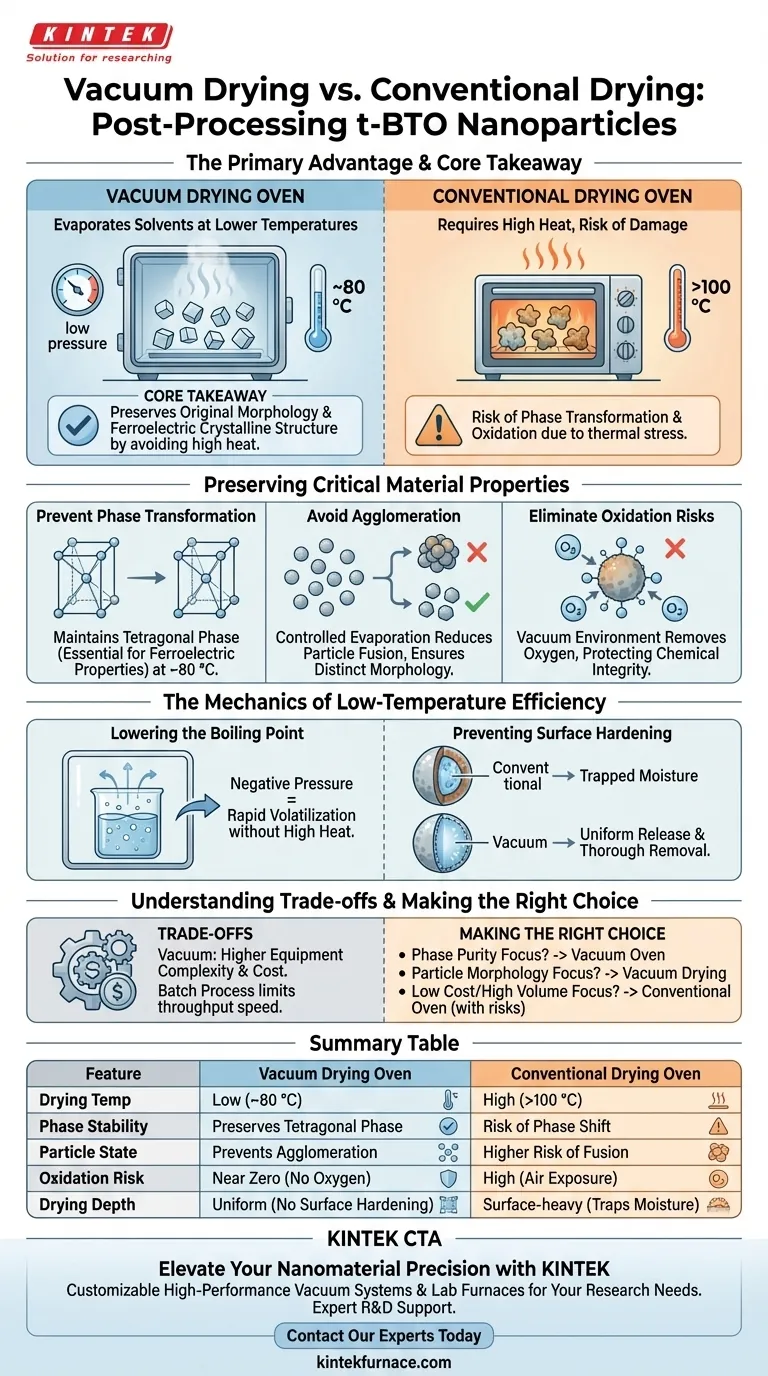
The primary advantage of using a vacuum drying oven for tetragonal barium titanate (t-BTO) nanoparticles is the ability to evaporate solvents at significantly lower temperatures. By lowering the ambient pressure, vacuum drying allows moisture and organic solvents to be removed at approximately 80 °C, avoiding the damaging high heat required by conventional ovens.
Core Takeaway: Conventional high-temperature drying poses a risk of altering the fundamental structure of t-BTO nanoparticles. Vacuum drying mitigates this by lowering the boiling point of solvents, effectively preserving the material's original morphology, preventing oxidation, and maintaining the critical ferroelectric crystalline structure.

Preserving Critical Material Properties
Preventing Phase Transformation
The tetragonal phase of barium titanate is essential for its ferroelectric properties. High temperatures in a conventional oven can inadvertently trigger a phase transformation, altering the crystal structure.
Vacuum drying operates effectively at lower temperatures (e.g., 80 °C). This ensures the nanoparticles remain in the desired tetragonal phase without thermal distortion.
Avoiding Agglomeration
When nanoparticles are dried at high atmospheric temperatures, the rapid evaporation and thermal energy can cause particles to fuse together.
Vacuum drying promotes a more controlled evaporation process. This significantly reduces the risk of agglomeration, ensuring the nanoparticles remain distinct and retain their original morphology.
Eliminating Oxidation Risks
Standard ovens expose materials to heated air, which increases the likelihood of oxidation.
The vacuum environment removes oxygen from the chamber. This protects the chemical integrity of the t-BTO nanoparticles, preventing surface oxidation that could degrade performance.
The Mechanics of Low-Temperature Efficiency
Lowering the Boiling Point
The defining feature of a vacuum oven is its ability to reduce the boiling point of liquids.
Under negative pressure, solvents like water or organic compounds volatilize rapidly without requiring high heat. This allows for deep drying of the material without subjecting it to thermal stress.
Preventing Surface Hardening
In conventional drying, high heat can cause the surface of a sample to dry and harden too quickly, potentially trapping moisture inside.
Vacuum drying facilitates a more uniform release of solvents. This ensures thorough removal of residuals from the nanoparticle structure, rather than just drying the outer surface.
Understanding the Trade-offs
Equipment Complexity and Cost
While vacuum drying yields superior material quality, it requires more complex equipment than a standard convection oven.
Users must manage vacuum pumps and ensure airtight seals. This adds a layer of maintenance and initial capital cost that is not present with simple thermal ovens.
Throughput Considerations
Vacuum drying is typically a batch process.
Unlike continuous conveyor ovens used in some large-scale industrial drying, vacuum ovens require the chamber to be sealed and depressurized for every batch. This can limit throughput speed in high-volume manufacturing scenarios.
Making the Right Choice for Your Goal
- If your primary focus is Phase Purity: Use a vacuum oven to ensure the t-BTO retains its ferroelectric tetragonal structure by keeping processing temperatures around 80 °C.
- If your primary focus is Particle Morphology: Choose vacuum drying to prevent hard agglomeration, ensuring the nanoparticles remain discrete and dispersible.
- If your primary focus is Low Cost/High Volume: A conventional oven may suffice, but only if the specific application can tolerate potential phase shifts or particle clumping.
Vacuum drying is the definitive choice for high-performance applications where the structural and chemical integrity of the t-BTO nanoparticle is non-negotiable.
Summary Table:
| Feature | Vacuum Drying Oven | Conventional Drying Oven |
|---|---|---|
| Drying Temp | Low (~80 °C) | High (>100 °C) |
| Phase Stability | Preserves Tetragonal Phase | Risk of Phase Shift |
| Particle State | Prevents Agglomeration | Higher Risk of Fusion |
| Oxidation Risk | Near Zero (No Oxygen) | High (Air Exposure) |
| Drying Depth | Uniform (No Surface Hardening) | Surface-heavy (Traps Moisture) |
Elevate Your Nanomaterial Precision with KINTEK
Don't compromise the ferroelectric integrity of your barium titanate. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum systems and lab high-temp furnaces, all customizable for your unique material needs. Whether you require precise temperature control for t-BTO or specialized atmospheres for advanced CVD/Rotary processes, our team ensures your research is supported by industry-leading equipment.
Ready to optimize your drying process? Contact our experts today to discuss your application and find the perfect furnace solution.
Visual Guide
References
- Rui Li, Shi Chen. Ferroelectricity enhances ion migration in hard carbon anodes for high-performance potassium ion batteries. DOI: 10.1039/d4nr04916k
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Induction Melting Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What are the technical advantages of using a vacuum drying oven? Protect WC-Co-Ni Powders from Oxidation
- What is the difference between a muffle furnace and a vacuum furnace? Choose the Right Heat for Your Process
- What are some common applications of graphite in vacuum furnaces? Essential for High-Temp Processing
- How does a vacuum annealing furnace reduce pollution? Achieve Cleaner Metal Processing with Zero Oxidation
- What are the specifications for resistance heating in vacuum graphitizing furnaces? Achieve Superior Graphitization for Large-Scale Production
- How does the evacuation process work in a vacuum furnace? Achieve Precise Metallurgical Control
- What is a vacuum furnace and how does it differ from an atmosphere furnace? Choose the Right Heat Treatment for Your Lab
- Why is a laboratory high-temperature furnace equipped with a high-vacuum system essential for metal-slag reactions?



















