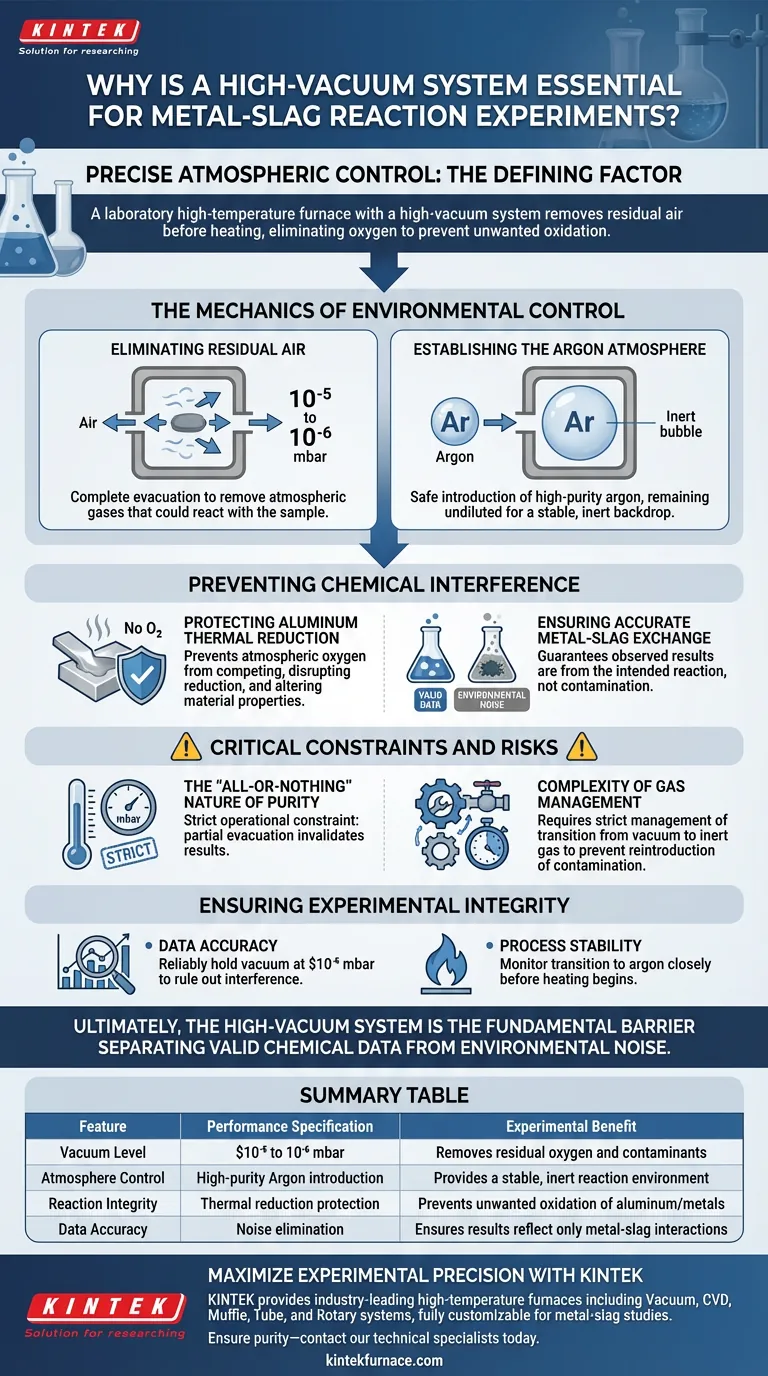
Precise atmospheric control is the defining factor in the success of metal-slag reaction experiments. A laboratory high-temperature furnace equipped with a high-vacuum system is essential because it removes residual air from the chamber before the heating process begins. This elimination of oxygen is critical to prevent unwanted oxidation, ensuring that chemical interactions occur solely between the metal and the slag.
The high-vacuum system acts as a prerequisite for purity, reducing chamber pressure to between $10^{-5}$ and $10^{-6}$ mbar to strip away environmental contaminants. This baseline allows for the subsequent introduction of high-purity argon, creating a strictly controlled environment that prevents atmospheric interference during thermal reduction.
The Mechanics of Environmental Control
Eliminating Residual Air
The primary function of the high-vacuum system is the complete evacuation of the furnace chamber.
Before any heating occurs, the system must achieve a vacuum level of $10^{-5}$ to $10^{-6}$ mbar.
This deep vacuum ensures that the baseline environment is virtually free of atmospheric gases that could later react with the sample.
Establishing the Argon Atmosphere
Once the residual air is removed, the vacuum system enables the safe introduction of high-purity argon gas.
Because the chamber was first evacuated, the introduced argon remains pure and is not diluted by remaining air pockets.
This creates the stable, inert backdrop required for the reaction phase.
Preventing Chemical Interference
Protecting Aluminum Thermal Reduction
In experiments involving the thermal reduction of aluminum, the presence of oxygen is detrimental.
The vacuum system ensures that atmospheric oxygen is not present to compete with or disrupt the reduction process.
Without this step, oxygen would react with the aluminum, skewing the experimental data and altering the material properties.
Ensuring Accurate Metal-Slag Exchange
The goal of these experiments is often to observe the chemical exchange between metal and calcium silicate slag.
Any interaction with atmospheric elements would create "noise" in the chemical data, making it impossible to isolate the metal-slag reaction.
The high-vacuum setup guarantees that the observed results are a product of the intended reaction, not environmental contamination.
Critical Constraints and Risks
The "All-or-Nothing" Nature of Purity
The requirement for such high vacuum levels ($10^{-5}$ mbar) introduces a strict operational constraint.
If the system fails to reach this specific pressure range, the integrity of the entire experiment is compromised.
Partial evacuation is insufficient; even trace amounts of residual air can invalidate the results of a sensitive metal-slag reaction.
Complexity of Gas Management
Using a high-vacuum system increases the complexity of the experimental setup compared to standard furnaces.
Operators must strictly manage the transition from vacuum to inert gas (argon) to maintain the protective atmosphere.
Failure to sequence these steps correctly will reintroduce contamination immediately before the reaction phase.
Ensuring Experimental Integrity
To obtain reliable data from your metal-slag reactions, consider the following recommendations:
- If your primary focus is data accuracy: Ensure your system can reliably hold a vacuum of at least $10^{-5}$ mbar to rule out oxidative interference.
- If your primary focus is process stability: Monitor the transition from vacuum to argon closely to ensure the inert atmosphere is established before heating begins.
Ultimately, the high-vacuum system is not just an accessory; it is the fundamental barrier that separates valid chemical data from environmental noise.
Summary Table:
| Feature | Performance Specification | Experimental Benefit |
|---|---|---|
| Vacuum Level | $10^{-5}$ to $10^{-6}$ mbar | Removes residual oxygen and contaminants |
| Atmosphere Control | High-purity Argon introduction | Provides a stable, inert reaction environment |
| Reaction Integrity | Thermal reduction protection | Prevents unwanted oxidation of aluminum/metals |
| Data Accuracy | Noise elimination | Ensures results reflect only metal-slag interactions |
Maximize Experimental Precision with KINTEK
Don't let atmospheric contamination compromise your research data. KINTEK provides industry-leading laboratory high-temperature furnaces, including Vacuum, CVD, Muffle, Tube, and Rotary systems, all specifically engineered to meet the rigorous demands of metal-slag and thermal reduction studies.
Backed by expert R&D and world-class manufacturing, our systems are fully customizable to your unique vacuum and atmospheric requirements. Ensure your next experiment is defined by purity—contact our technical specialists today to discuss your custom solution.
Visual Guide
References
- Harald Philipson, Kristian Etienne Einarsrud. Investigation of Liquid–Liquid Reaction Phenomena of Aluminum in Calcium Silicate Slag. DOI: 10.3390/ma17071466
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the classification of a vacuum furnace? Match Performance, Process & Temperature to Your Needs
- What support services are available for custom vacuum furnace users? Ensure Lifelong Performance and Uptime
- What is the heat treatment in a vacuum furnace? Achieve Superior Metallurgical Properties
- How does a sintering furnace work? Master the Process for Superior Material Properties
- Why does the simulation of magnesium alloy distillation require high precision? Master Vacuum for Purity
- How does a vacuum annealing furnace modify the bond coat? Optimize TBC Systems for Superior Thermal Protection
- What are the limitations of high vacuum furnaces? Understand Costs, Time, and Material Challenges
- What is the difference between an atmosphere furnace and a vacuum furnace? Choose the Right Heat Treatment for Your Lab



















