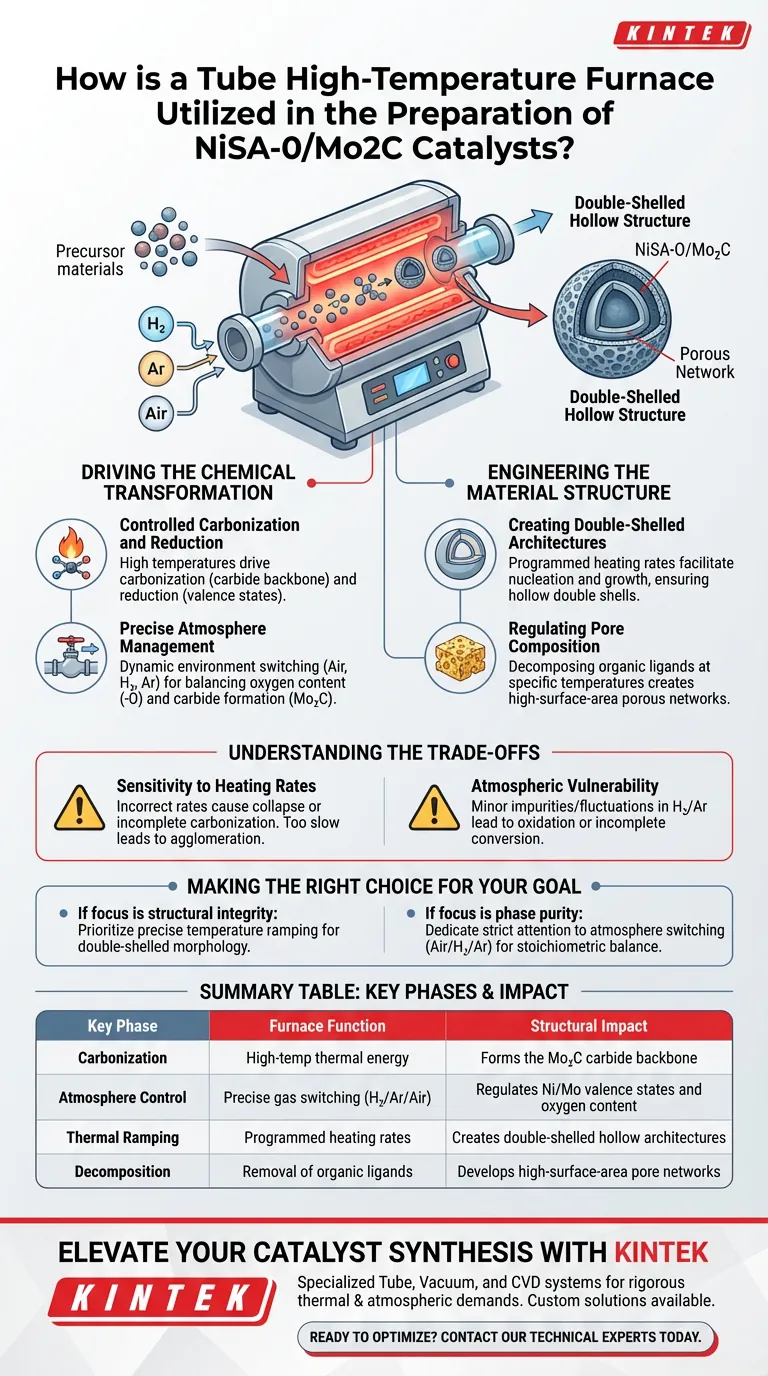
The tube high-temperature furnace functions as the primary reactor for the critical carbonization and reduction phases required to synthesize NiSA-O/Mo2C catalysts. It utilizes programmed temperature ramping and precise atmosphere switching to facilitate the in situ transformation of precursors into molybdenum-based carbides featuring unique double-shelled hollow structures.
The tube furnace provides the essential combination of thermal energy and environmental stability needed to regulate the material's phase composition and pore structure, turning raw precursors into a highly active catalytic architecture.

Driving the Chemical Transformation
Controlled Carbonization and Reduction
The central role of the furnace is to drive the chemical conversion of precursor materials. By subjecting the material to high temperatures, the furnace initiates the carbonization process, which forms the carbide backbone of the catalyst. Simultaneously, it manages reduction tasks to ensure the correct valence states of the metal components.
Precise Atmosphere Management
The synthesis of NiSA-O/Mo2C requires a dynamic environment. The tube furnace allows operators to switch seamlessly between different gas atmospheres, such as air, hydrogen, and argon. This control is vital for defining the chemical nature of the catalyst, specifically balancing the oxygen content (the "-O" component) and the carbide formation (Mo2C).
Engineering the Material Structure
Creating Double-Shelled Architectures
The primary reference highlights that this specific catalyst possesses a "double-shelled hollow structure." The tube furnace facilitates this morphology through carefully programmed heating rates. The thermal treatment dictates how the material nucleates and grows, preventing collapse and ensuring the formation of these complex shells.
Regulating Pore Composition
Beyond the macro-shape, the furnace determines the internal porosity of the material. By decomposing organic ligands within the precursors at specific temperatures, the furnace creates a porous network. This high surface area is critical for exposing the active sites of the catalyst to reactants during its final application.
Understanding the Trade-offs
Sensitivity to Heating Rates
While the furnace allows for programmed ramping, incorrect heating rates can be detrimental. Rapid heating may cause structural collapse or incomplete carbonization, destroying the desired double-shelled morphology. Conversely, heating that is too slow may lead to excessive particle agglomeration, reducing catalytic surface area.
Atmospheric Vulnerability
The quality of the final Mo2C phase is strictly dependent on the purity and flow of the reduction gases. Even minor fluctuations in the hydrogen or argon atmosphere during the reduction phase can lead to unwanted oxidation or incomplete conversion, rendering the catalyst less effective.
Making the Right Choice for Your Goal
To optimize the preparation of NiSA-O/Mo2C catalysts using a tube furnace:
- If your primary focus is structural integrity: Prioritize the precision of your temperature ramping program to preserve the double-shelled hollow morphology.
- If your primary focus is phase purity: Dedicate strict attention to the atmosphere switching protocols (Air/H2/Ar) to ensure the correct stoichiometric balance of the carbide and oxide components.
Success in synthesizing this catalyst relies not just on reaching high temperatures, but on the precise orchestration of thermal and atmospheric cycles.
Summary Table:
| Key Phase | Furnace Function | Structural Impact |
|---|---|---|
| Carbonization | High-temp thermal energy | Forms the Mo2C carbide backbone |
| Atmosphere Control | Precise gas switching (H2/Ar/Air) | Regulates Ni/Mo valence states and oxygen content |
| Thermal Ramping | Programmed heating rates | Creates double-shelled hollow architectures |
| Decomposition | Removal of organic ligands | Develops high-surface-area pore networks |
Elevate Your Catalyst Synthesis with KINTEK
Precision is the difference between a collapsed structure and a high-performance NiSA-O/Mo2C catalyst. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Tube, Vacuum, and CVD systems designed for the rigorous thermal and atmospheric demands of modern material science.
Our lab high-temp furnaces provide the advanced temperature ramping and gas management systems essential for engineering complex double-shelled architectures. Whether you need a standard setup or a fully customizable solution tailored to your unique research needs, KINTEK delivers the reliability your lab deserves.
Ready to optimize your catalytic architecture? Contact our technical experts today and let us help you build the perfect furnace for your application.
Visual Guide
References
- Mengyun Hou, Chen Chen. Microenvironment reconstitution of highly active Ni single atoms on oxygen-incorporated Mo2C for water splitting. DOI: 10.1038/s41467-024-45533-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the key features of tube furnaces? Unlock Precision in High-Temperature Processing
- What are the advantages of different heating zone configurations in tube furnaces? Optimize Your Thermal Processes
- What are the temperature control requirements for SiC@SiO2 in-situ oxidation? Achieve Precise 1100°C Thermal Stability
- How does the temperature control program of a tube furnace affect NiSSe nanocrystal formation? Optimize Your Synthesis
- What is the significance of temperature zoning for 1D ZnSe nanowires? Master Thermal Gradients for VLS Growth
- How are vertical fluidized bed tube furnaces utilized in material handling and processing? Achieve Uniform Thermal Processing for Powders
- What are the space-saving benefits of a tube furnace? Maximize Lab Efficiency with Compact Design
- What are the essential functional requirements for a tube furnace used in the biomass activated carbon activation process?



















