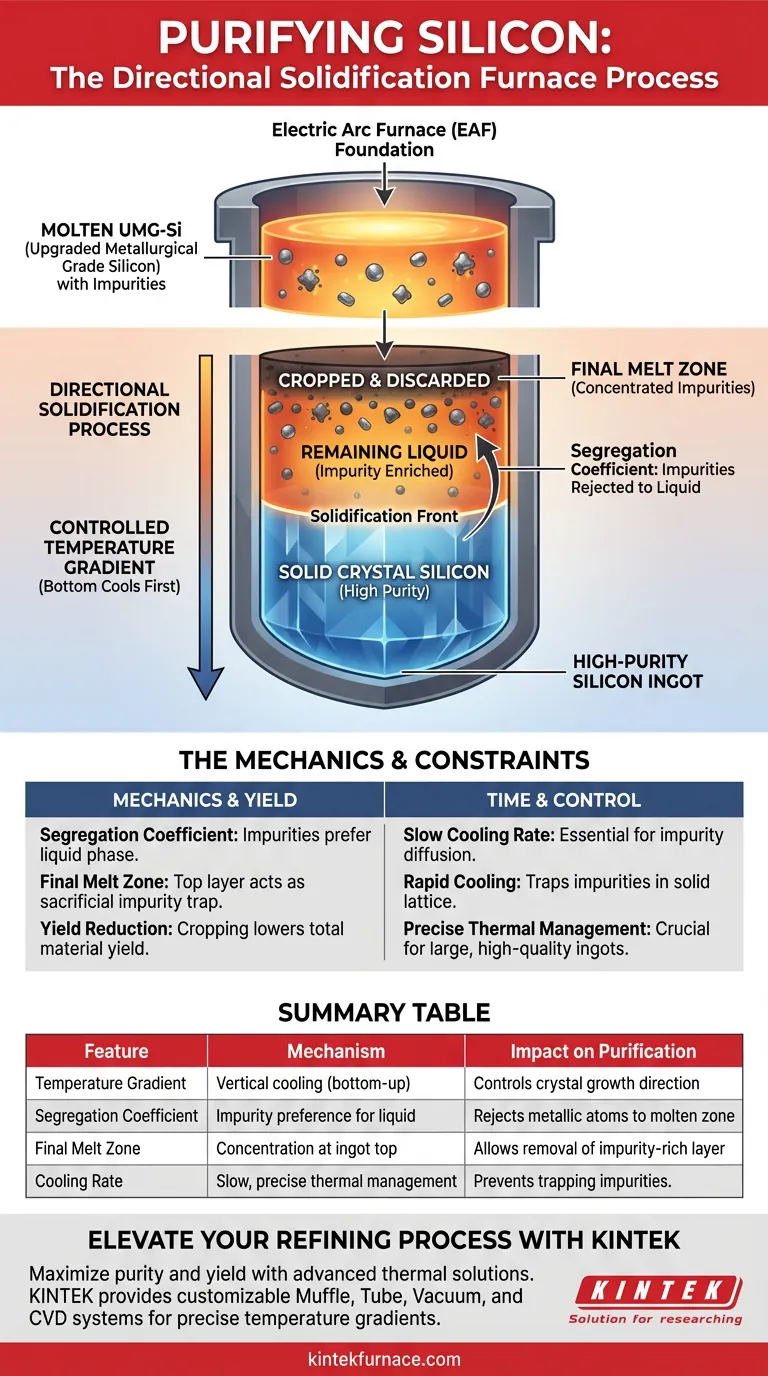
A directional solidification furnace purifies upgraded metallurgical grade silicon (UMG-Si) by exploiting the solubility differences of impurities during cooling. By establishing a precise temperature gradient, the furnace forces the silicon to crystallize slowly from the bottom of the crucible upward, effectively separating pure silicon from metallic contaminants.
The core mechanism relies on the segregation coefficient of metallic impurities. Because these impurities prefer to remain in the liquid molten state rather than the solid crystal structure, they are continuously rejected by the solidifying silicon and pushed upward into the final "melt zone" at the top of the ingot.

The Mechanics of Purification
Controlled Temperature Gradients
The furnace does not cool the silicon uniformly. Instead, it maintains a strict temperature gradient that ensures the bottom of the crucible cools first.
This allows the solidification front to move vertically—from the bottom to the top—in a controlled manner.
The Role of Segregation Coefficients
The chemical principle driving this purification is the difference in segregation coefficients between solid and liquid phases.
Metallic impurities have a much higher solubility in liquid silicon than in solid silicon. Consequently, as the silicon atoms lock into a crystal lattice, they reject the foreign metallic atoms.
Concentration in the Final Melt Zone
As the solidification front advances upward, the concentration of rejected impurities in the remaining liquid increases.
Eventually, the majority of these metallic impurities are trapped in the very top layer of the ingot, known as the final melt zone. This allows the bulk of the ingot below to remain highly pure.
Contextualizing the Process
The Foundation: Electric Arc Furnace
It is important to understand where this fits in the production chain. The Electric Arc Furnace (EAF) serves as the initial foundation.
The EAF facilitates the reduction reactions that create the initial metallurgical grade silicon. Directional solidification then acts as the subsequent refining step to remove the metallic impurities left behind by the EAF process.
Understanding the Constraints
Yield vs. Purity
While effective, this process creates a necessary waste product.
Because the impurities are concentrated at the top of the ingot, this section acts as a "sacrificial" layer. The top portion must be mechanically removed (cropped) and discarded to access the high-purity silicon beneath, resulting in a reduction of total material yield.
Time and Control
The effectiveness of impurity removal is directly tied to the speed of solidification.
If the cooling is too rapid, the impurities will not have time to diffuse into the liquid and will be trapped within the solid crystal. Therefore, the process requires patience and precise thermal management to ensure large-area, high-quality ingots.
Making the Right Choice for Your Goal
To maximize the effectiveness of directional solidification, you must view it as part of a larger system.
- If your primary focus is maximizing purity: Ensure your thermal control system maintains a slow, stable solidification front to prevent impurity trapping.
- If your primary focus is process efficiency: Optimize the feedstock quality from the Electric Arc Furnace stage to minimize the initial impurity load before solidification begins.
Success lies in balancing the speed of the temperature gradient with the physical limitations of impurity segregation.
Summary Table:
| Feature | Mechanism | Impact on Purification |
|---|---|---|
| Temperature Gradient | Vertical cooling from bottom to top | Controls the direction of crystal growth |
| Segregation Coefficient | Impurity preference for liquid phase | Rejects metallic atoms into the molten zone |
| Final Melt Zone | Concentration at the ingot top | Allows easy removal of the impurity-rich layer |
| Cooling Rate | Slow, precise thermal management | Prevents trapping impurities in the solid lattice |
Elevate Your Silicon Refining Process with KINTEK
Maximize your material purity and yield with advanced thermal solutions from KINTEK. As experts in high-temperature laboratory systems, we understand that precision is the key to effective directional solidification.
Whether you are refining metallurgical grade silicon or developing next-generation semiconductor materials, KINTEK provides the high-performance Muffle, Tube, Vacuum, and CVD systems you need. Our furnaces are fully customizable and backed by expert R&D to meet your specific temperature gradient requirements.
Ready to optimize your purification workflow? Contact us today to discuss how our laboratory high-temp furnaces can bring superior control and efficiency to your lab.
Visual Guide
References
- Production of upgraded metallurgical-grade silicon for a low-cost, high-efficiency, and reliable PV technology. DOI: 10.3389/fphot.2024.1331030
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does the vacuum environment in these furnaces improve metal quality? Achieve Superior Purity and Strength
- How is furnace brazing applied in the aerospace industry? Join High-Performance Components with Precision
- What are the core principles of vacuum furnace engineering? Master Precision Control for Superior Materials
- How do computer-controlled systems enhance vacuum furnace operations? Achieve Precision and Repeatability in Heat Treatment
- What challenges does vacuum brazing pose for the vacuum system? Master Gas Load Management for Flawless Joints
- What is the core role of a vacuum melting furnace in the process of recovering elemental magnesium from slag? | Achieve High-Purity Metal Recovery
- What are the advantages of using a vacuum oven for drying NiFe2O4/biochar samples? Preserve Purity and Porosity
- What role do high-temperature melting furnaces play in Al-6.8Zn-2Mg-2Cu-0.1Zr-0.2Sc alloys? Optimize Alloy Homogeneity



















